Metallo-supramolecular block copolymers
We are
synthesizing and characterizing a new type of amphiphilic block
copolymers, namely metallo-supramolecular amphiphilic block
copolymers. These compounds are formed by a hydrophilic
poly(ethylene oxide) A block linked to a hydrophobic B block such
as poly(styrene) through a
bis-2,2’:6’,2’’-terpyridine-ruthenium(II)
complex. The terpyridine ligand can easily be introduced at the
chain end of different polymers, thus giving rise to many possible
combinations of different blocks. Since the method for preparing
block copolymers relies on the coupling of two polymer chains via a
simple two-step synthesis using principles from coordination
chemistry around a ruthenium metal ion, it is straightforward to
prepare a library of block copolymers. Compared to
“classical” covalent block copolymers,
metallo-supramolecular block copolymers offer many advantages.
Besides the formation via self-organization processes, the
reversibility of the supramolecular bond allows the construction of
“smart materials” with tunable properties. Moreover,
the electrochemical and photochemical properties of the complexes
can be engineered by choosing the appropriate metal ion and
counter-ion. Recently, we focused on a library of
metallo-supramolecular block copolymers composed of poly(styrene)
and poly(ethylene oxide) joined by a bis-terpyridine-ruthenium
complex. The micelles formed from these copolymers have been
characterized by AFM and TEM. The results evidence that the
classical scaling laws relating the micelle size to the size of the
hydrophobic block are not valid for metallo-supramolecular
copolymers. This different behavior is due to electrostatic
repulsions between the charged and bulky metal-ligand complexes
present at the core-corona interface. If those repulsions are
screened by increasing the ionic strength during micelle formation,
the classical behavior is observed.
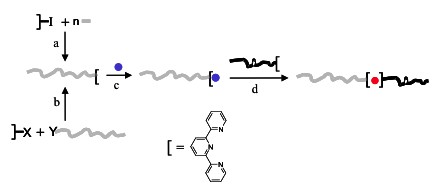
Schematic
representation of the synthetic strategy to prepare linear
metallo-supramolecular block copolymers. The first step is the
synthesis of the macroligands by either using a functionalized
(supramolecular) initiator (a), or grafting a terpyridine on a
reactive chain end (b). The second step is the preparation of a
mono-complex with RuIII (c).
Finally, the second block, bearing a free terpyridine unit, is
added to form a terpyridine-ruthenium(II) bis-complex
(d).
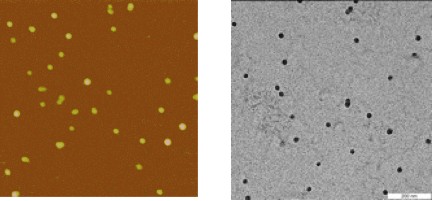
AFM
(left) and TEM (right) pictures recorded on micelles prepared from
the PS200-[Ru]-PEO375
copolymer in
water. AFM image is 11 µm.
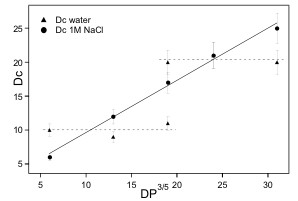
Relationship
between the measured (AFM) core size of the micelles and the
3/5th power of the
PS block DP, for micelles prepared in pure water (triangles) and in
1M NaCl (circles). The solid line represents the linear regression
obtained from the data in 1M NaCl.
Researchers
involved: Pierre Guillet, Clément Mugemana
Collaborations:
Ulrich Schubert
(Eindhoven)
Relevant
papers:
"Study of the influence of the metal-ligand complex on the size of
aqueous metallo-supramolecular micelles"
P. Guillet, C.-A. Fustin, B. G. G. Lohmeijer, U. S. Schubert, J.-F.
Gohy
Macromolecules
2006, 39,
5484-5488
“Metallo-supramolecular block copolymers”
P. Guillet, C.-A. Fustin, U. S. Schubert, J.-F.
Gohy
Adv.
Mater.
2007, 19, 1665-1673