Micellization of oxazoline-based block
copolymers
Block copolymer
micelles have found widespread applications ranging from coatings,
cosmetics, oil recovery and bio-related systems. For the
application of polymers in, e.g., drug delivery, other
characteristics such as the polymer’s biocompatibility and
degradability have to be considered as well. This biocompatibility
directed us to the class of poly(2oxazoline)s with its numerous
congeners, among which the poly(2-ethyl-2oxazoline) has been FDA
approved. The 2-oxazoline monomers undergo living cationic
ring-opening polymerization under the appropriate conditions
resulting in well-defined polymers with narrow molecular weight
distributions. Nevertheless, the polymerizations normally require
reaction times in the range of several hours up to several days.
This disadvantage was recently overcome in Prof. Ulrich
Schubert’s group by the use of closed reaction vials and
microwave irradiation6 that accelerated the cationic ring-opening
polymerization of 2-oxazolines by a factor of 400 when compared to
conventional reflux polymerizations. This observed acceleration was
found to solely originate from thermal effects and not from
so-called (non-thermal) microwave effects. However, slightly
narrower molecular weight distributions were obtained under
microwave heating due to the fast heating and the homogeneous heat
profile in the reaction vessel. This improved microwave
polymerization procedure was recently applied to the synthesis of
libraries of diblock, triblock and tetrablock copoly(2-oxazoline)s
based on 2-methyl- (MeOx), 2-ethyl- (EtOx),
2-“soy”oxazoline (SoyOx) and 2-phenyl-2-oxazoline
(PhOx). The micellization behavior of block copoly(oxazolines) of
AB, ABC, ABCA, ABCB and ABCD architecture is currently investigated
in our group.
PEtOx-PSoyOx diblock copolymers have been used to prepare aqueous
spherical micelles consisting of a PEtOx corona and a PSoyOx core,
which has been further crosslinked by UV-irradiation. The
morphology of these crosslinked micelles has been shown to
reversibly change from spheres to short rods referred to as rice
grain whenever the micelles were transferred from water into
acetone, a non-selective solvent for the constituent blocks. This
morphological transition has been attributed to the swelling of the
slightly crosslinked PSoyOx core.
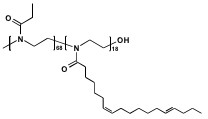
Chemical
Structure of the investigated PEtOx-PSoyOx diblock
copolymer.
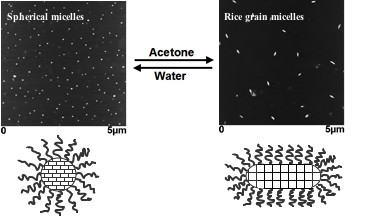
Reversible
morphological transition observed in core-crosslinked PEtOx-PSoyOx
micelles.
The formation of
micelles on surfaces by spin-coating dilute solutions of diblock,
triblock, and tetrablock copoly(2-oxazoline)s in a non-selective
has been also demonstrated. Those micelles are not pre-existing in
the initial solution but are formed during the evaporation of the
solvent by the precipitation of the least soluble block. The
morphology and size of the micelles vary according to the fraction
of this block but is not dependent on the block order in the
copolymer.
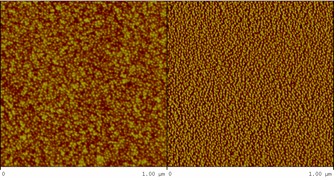
AFM
image (left: height image, right: phase image) of a spin-coated
sample from a 1 g/L solution of the
MeOx25-EtOx25-PhOx25-EtOx25
copolymer in
ethanol.
Researchers involved: Haying Huang, Nathalie Lefèvre
Collaborations:
Ulrich Schubert
(Eindhoven), Richard Hoogenboom (Eindhoven), Alain Jonas
(UCL)
Relevant
papers:
"Solvent-induced morphological transition in core-crosslinked block
copolymer micelles"
H. Huang, R. Hoogenboom, M. A. N. Leenen, P. Guillet, A. Jonas, U.
S. Schubert, J.-F. Gohy
J. Am. Chem.
Soc.
2006, 125,
3784-3788
"Aqueous micelles formed by ABC, ACB, ABCA and ABCB
copoly(2oxazoline)s"
J.-F. Gohy, H. Huang, C.-A. Fustin, A. M. Jonas, R. Hoogenboom, M.
A. M. Leenen, F. Wiesbrock, H. M. L. Thijs, S. F. G. M. van Nispen,
M. van der Loop, U. S. Schubert
Polymer
Preprints
2006, 47,
745-746
"Microwave-assisted ring-opening cationic polymerization of
2-oxazolines: a powerful method for the synthesis of amphiphilic
triblock terpolymers"
R. Hoogenboom, F. Wiesbrock, H. Huang, M. A. M. Leenen, H. M. L.
Thijs, S. F. G. M. van Nispen, M. van der Loop, C.-A. Fustin, A.M.
Jonas, J.-F. Gohy, U.S. Schubert
Macromolecules
2006, 39,
4719-4725
"Microwave-assisted synthesis and micellization behavior of
soy-based copoly(2-oxazoline)s"
R.Hoogenboom, M. A. M. Leenen, H. Huang, C.-A. Fustin, J.-F. Gohy,
U. S. Schubert
Colloid
polym. Sci.
2006, 284,
1313-1318
"Evaporation induced micellization of poly(2-oxazoline) multiblock
copolymers on surfaces"
C.-A. Fustin, H. Huang, R. Hoogenboom, F. Wiesbroek; A. M. Jonas,
U. S. Schubert, J.-F. Gohy
Soft
Matter
2007, 3, 79-82
"Synthesis and Aqueous Micellization of Amphiphilic Tetrablock Ter- and Quaterpoly(2-oxazoline)s"
R. Hoogenboom, F. Wiesbrock, M. A. M. Leenen, H. M. L. Thijs, H.
Huang, C.-A. Fustin, P. Guillet, J.-F. Gohy, U. S.
Schubert
Macromolecules
2007, 40, 2837-2843