Hydrogen-bonded complexes in block copolymers
In order to
broaden the range of micellar structures and functionalities,
specific non-covalent interactions have been recently considered as
driving force for micellization. In this respect, non-covalent
interactions between mutually interacting polymer blocks or between
a polymer block and low molecular weight functional molecules such
as surfactants have been used. The complexes resulting from these
non-covalent interactions may be insoluble and further aggregate
into a micellar core. These micellar cores are surrounded by a
corona formed by the polymer blocks which were not involved in the
complexation process.
We use this strategy to prepare micelles in organic solvents from
poly(styrene)-block-poly(4-vinylpyridine) (PS-b-P4VP) diblock
copolymers mixed with either fluorinated surfactants bearing a
carboxylic acid group or poly(acrylic acid) (PAA). In both cases,
hydrogen-bonding between the P4VP blocks and carboxylic acid groups
is the driving force for aggregation.
Spherical micellar aggregates have been obtained in chloroform by
mixing PS-b-P4VP diblock copolymers with perfluorinated surfactants
(FS) bearing a carboxylic acid head. These micellar aggregates are
resulting from the self-assembly of the insoluble P4VP/fluorinated
complexes into a core surrounded by the soluble PS coronal chains.
Their characteristic features have been studied as a function of
various parameters including the composition of the PS-b-P4VP
copolymer, the tail length of the fluorinated surfactant, the
4VP/FS molar ratio, the number of carboxylic acid group (1 or 2) on
the surfactant, the presence of the PS block and of the fluorine
atoms on the surfactant. Dilution of these initial micellar
aggregates triggers a morphological reorganization resulting in the
formation of more stable vesicles. The extent of this morphological
transition is related to the solubility of the P4VP/fluorinated
complexes during the dilution process. This transition is complete
for short P4VP/FS complexes, incomplete for long P4VP/FS complexes
and is not observed whenever an α,ω-difunctional
FS is used in P4VP/FS complexes, leading to a crosslinked core.
This spheres-to-vesicles transition has been advantageously used in
order to encapsulate molecules, as demonstrated by confocal
fluorescence microscopy.
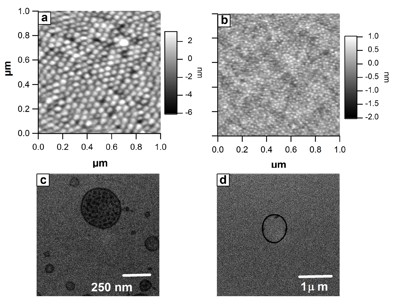
Micellar
aggregates initially formed at a concentration of 1 g/L by (a)
PS192-b-P4VP179/PFPA
and (b) PS327-b-P4VP27/PFPA
and vesicles observed after dilution at 0.1 g/L by (c)
PS192-b-P4VP179/PFPA
and (d) PS327-b-P4VP27/PFPA
(4VP/PFPA molar ratio of 4/1 in all cases; a and b: AFM height
contrast pictures; c and d: TEM pictures).
Micelles
have been prepared by mixing
poly(styrene)-block-poly(4-vinylpyridine) (PS-b-P4VP) copolymers
and poly(acrylic acid) (PAA) homopolymers in organic solvents.
Complexation via hydrogen bonding occurs between the P4VP and PAA
blocks. Further aggregation of the accordingly formed complexes
results in micelles stabilized by a corona of PS blocks. The
influence of the relative lengths of the different blocks and of
the quality of the solvent towards the complexes on the micellar
characteristic features is studied. Soluble complexes have been
observed in DMF, provided that the complexes are sufficiently
small. In all other cases, micelles have been obtained. The size of
the those micelles depends strongly on the length of the P4VP
blocks but only weakly on the PAA length. Reorganization of these
structures occurs upon addition of acidic or basic water, which
results in the breaking of the hydrogen bonds between the P4VP and
PAA blocks. After transfer of the initial complexes in acidic
water, micelles consisting of a PS core and a protonated P4VP
corona are observed. In basic water, well-defined nanoparticles
formed by the PS-b-P4VP copolymers are obtained. It is demonstrated
that these nanoparticles are stabilized by the negatively charged
PAA chains. Finally, thermally-induced disintegration of the
micelles is investigated in organic
solvents.
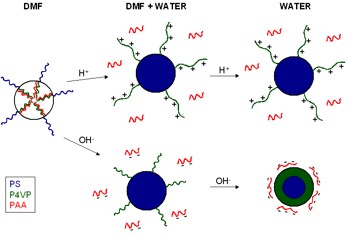
Reorganization
of the micellar structure due to the addition of acidic or basic
water in PS-b-P4VP/PAA mixtures.
Researchers involved: Zhijun Hu, Nathalie Lefèvre
Collaborations:
Alain Jonas
(UCL), Johan Hofkens (KUL)
Relevant
papers:
"Dilution-induced spheres-to-vesicles morphological transition in
micelles from block copolymer/surfactant complexes"
Z. Hu, S. Varshney, A. M. Jonas, J.-F.
Gohy,
J. Am. Chem.
Soc.
2005, 127,
6526-6527
"Formation of vesicles in block copolymer-fluorinated surfactant
complexes"
Z. Hu, W. Verheijen, J. Hofkens, A. M. Jonas, J.-F.
Gohy
Langmuir
2007, 23,
116-122
"Reorganization of hydrogen bonded block copolymer complexes"
N. Lefèvre, C.-A. Fustin, J.-F. Gohy
Langmuir
2007, 23, 4618-4622