Contact

Croix du sud 4-5/L7.07.06
Carnoy b.222
1348 Louvain-la-Neuve
Phone: +32485232610
Research
Within the team of Prof. Patrice Soumillion my research centers on the engineering of new bioactive cyclic peptides. I use the SICLOPP (Split Intein–mediated Circular Ligation of Peptides and Proteins) technology coupled with a co-translational export peptide to produce large libraries of peptides in the periplasm of E. coli. Those libraries can then be mined to look for targets of interest, for example inhibitors of the enzymes involved in resistance to carbapenemases, or inhibitors of the cell wall biogenesis of Gram- bacterias.
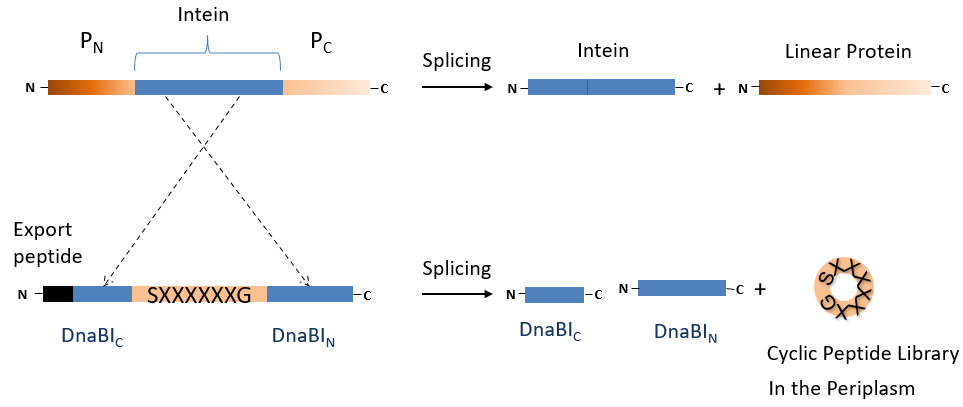
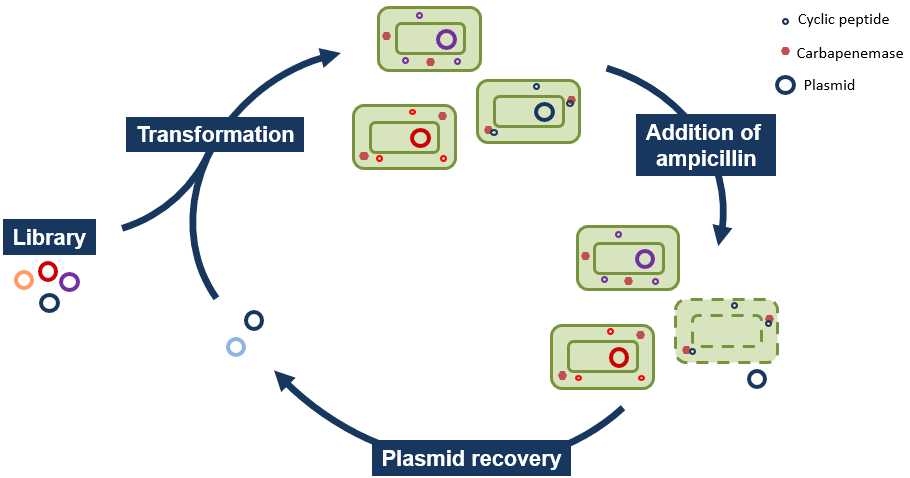
In order to identify the peptides of interest from the libraries, we rely on a technique that we called “Selection by Lysis” (See Fig. 2). This let us leverage the power of selection approach and their very high throughput. After transformation in a strain expressing our target carbapenemase, the cells are grown and an antibiotic such as ampicillin is added. Cells expressing an inhibitor will be sensitive and lyse, releasing their plasmids into the culture medium.
By centrifugating down the living cells and cell debris, we can recover the culture medium, from which the plasmids encoding the peptides of interest can be recovered and sequenced or retransformed for further selection.

 orcid.org/0000-0003-1186-0148
orcid.org/0000-0003-1186-0148