Asymmetric Redox Catalysis (former Phd
students Ahlem Bensari, James Courmarcel, Post doctoral research fellow Jérome
Hannedouche, Post graduate student Isabelle Bergiers)
The enantio-control of C-C bond formation with radical
anions is a challenging research area in which we have been involved
for the development of new useful tools for asymmetric construction of chiral
molecules. We have focussed our first efforts on the Enantioselective catalysis
of the pinacol reaction of aromatic aldehydes using in situ generated chiral
low valent titanium complexes.
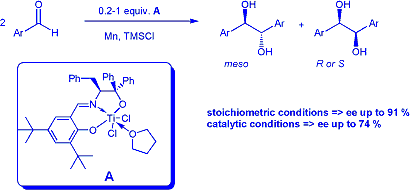
A straightforward and general method for the preparation of a
new family of air stable chiral titanium complexes (such as A) has
been designed. Enantioselectivities reaching 91 % in the case of aromatic
aldehydes have been reached after careful optimisation of the reaction conditions.
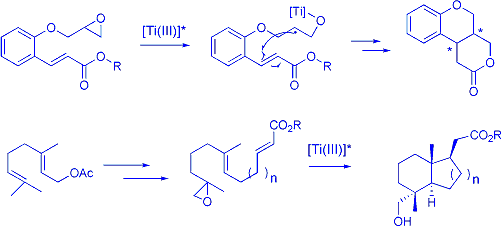
We are now involved in the generation of chiral -oxyradical anions which are
generated from epoxides. We are presently studying the
asymmetric polycyclisation of such radicals for the construction of
interesting polycyclic structures.
Asymmetric Hydrosilylation (Naouel
Mostefaï, former Post-docts Sabine Sirol and Sabine Chopin, former Phd
student James Courmarcel, Phd student Yann Pressel, post graduate student
David Leclerq).
The stereoselective synthesis of optically active alcohols is a well studied topic in organic chemistry. In area of research, the search for economical methods of enantioselective reduction of prochiral ketones is a rewarding goal. Polymethylhydrosiloxane (PMHS), a polymer co product of the silicone industry, is a safe and inexpensive reducing silylating agent which can transfer its hydride to a variety of metal catalysts (Ti, Sn, Zn, …) participating in a wide range of reductions.
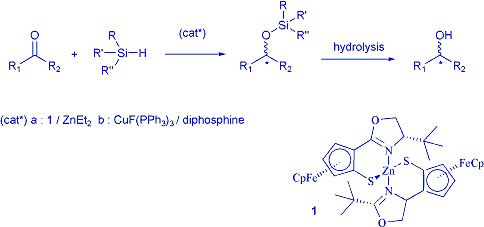
Our contribution to this area is focused on the discovery of new
efficient catalytic systems for the asymmetric hydrosilylation of prochiral
ketones. Two new systems have been found and are currently under optimisation
in our laboratory. The first system was inspired by the pioneer work of Mimoun
on the use of chiral zinc complexes as catalysts for hydrosilylation. Extension
of our studies on chiral ferrocenyl ligands prompted us to develop another
low cost transition metal catalytic system focusing on N,S-zinc catalysts.
The second system is based on the generation of chiral copper (I) hydrides
from an easily available organometallic precursor. An unusual acceleration
effect by oxygen was discovered and allowed the discovery of one of the fastest
hydrosilylation catalyst reported so far. Enantioselectivies exceeding 90
% were obtained for the hydrosilylation of prochiral ketones after careful
optimisation of the reaction conditions.
Design of new librairies of chiral ligands for high-throughput
screening (Phd student Nicolas Vriamont)
We are currently involved in collaboration with the group of Dr Claude de
Bellefon (CPE Lyon, France) regarding new methods for the high-throughput
screening of chiral catalysts. Our contribution is first focussed on the preparation
of libraries of chiral ligands by parallel synthesis. We are also interested
in a better understanding of the method used for the optimisation of organometallic
catalysts
Collaborations
Artificial metalloenzymes for asymmetric catalysis : collaboration with Profs Patrice Soumillion and Jacques Fastrez (Unité BIOC, UCL)
Asymmetric photodeprotection of tosylamides (Phd student Olivier Chuzel): Collaboration with Prof. Jean-Philippe Soumillion
Design of new librairies of chiral ligands for high-throughput screening (Phd student Nicolas Vriamont): Collaboration with Dr Claude de Bellefon (CPE Lyon, France)
Total synthesis of a natural product with anti-hypertensive activity : (Phd Student Julia Deschamp): Collaboration with Prof. Joelle Quetin-Leclercq (unité CHAM, UCL)
New methodologies for the asymmetric synthesis of chiral ligands (Phd student Patrick Pouplard) Collaboration with the Solvias company (Basel, Swizerland)