Thi7 endocytosis
Role of α-arrestin adaptors in yeast plasma membrane transporter endocytosis
Our lab is interested in membrane trafficking questions such as endocytosis. In the past, we developed an optimized plasma membrane purification procedure and a quantitative gel-free proteomic approach to monitor changes in the yeast plasma membrane proteome in cells exposed to external stress. With this approach, we are currently studying the role of the ubiquitin ligase Rsp5 and α-arrestin adaptors in endocytosis of several amino acid or vitamin transporters.
Using quantitative proteomics, we have recently identified new targets for α-arrestins and provided new insight into the diversity of pathways affected by them, such as the cell wall integrity pathway or the PM–endoplasmic reticulum (ER) tethering system. Among the identified targets, we are now focusing on the poorly characterized thiamine (vitamin B1) transporter Thi7. Thiamine is the precursor of thiamine pyrophosphate (TPP), a coenzyme involved in both carbohydrate and amino acid metabolism. Therefore, thiamine uptake through Thi7 has a global effect on cellular metabolism. We have described a new role for the Art2 α-arrestin as a mediator of the substrate- and stress-induced endocytosis of the thiamine transporter Thi7 (Fig 1). We are currently investigating the underlying mechanism of Thi7 endocytosis in response to thiamine transport.
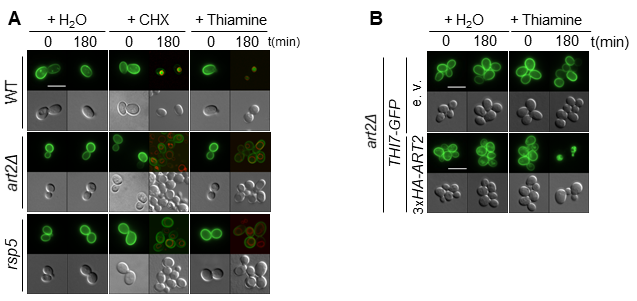
Figure 1 : The ubiquitin ligase Rsp5 and the Art2 α-arrestin are required for stress- (cycloheximide; CHX) and substrate- (thiamine) induced endocytosis of the vitamin B1 transporter Thi7. (A) Localization of Thi7-GFP in the WT, art2Δ, and rsp5 strains grown in thiamine-free medium after addition of thiamine or cycloheximide (CHX) or mock-treated with water. The vacuolar membrane is stained with FM4-64. (B) Expression of 3xHA-ART2 restores thiamine-induced endocytosis of Thi7-GFP in the art2Δ background.
Key publications :
- Szopinska et al. Rapid response of the yeast plasma membrane proteome to salt stress. Molecular and Cellular Proteomics, Vol. 10, no. 11, p. M111.009589 (2011). http://hdl.handle.net/2078.1/88839
- De Block et al. Yeast Pmp3p has an important role in plasma membrane organization. Journal of Cell Science, Vol. 128, no.19, p. 3646-3659 (2015). http://hdl.handle.net/2078.1/165661
- Villers et al. Study of the plasma membrane proteome dynamics reveals novel targets of nitrogen regulation in yeast. Molecular and Cellular Proteomics, Vol. 16, no. 9, p. 1652-1668 (2017). http://hdl.handle.net/2078.1/186819
- Savocco et al. Yeast α-arrestin Art2 is the key regulator of ubiquitylation-dependent endocytosis of plasma membrane vitamin B1 transporters. PLoS Biology, Vol. 17(10): e3000512, (2019), doi: 10.1371/journal.pbio.3000512.