BAR domain proteins
Role of BAR-domain proteins in endocytosis and mechanotransduction
Among the different mechanisms that cells developed to sense/induce changes in membrane geometry, one of the most fascinating cases is the superfamily of proteins containing a BAR (Bin/Amphiphysin/Rvs) domain. Members of this protein family (64 in Homo sapiens) are composed of a three-helix coiled coil core that assembles into a six-helix curved dimer about 20 nm long. These dimers are the functional unit and share a characteristic curved banana-like shape. These proteins are key players in a variety of cellular processes such as endocytosis, intracellular vesicle formation, actin regulation and cell migration to name only a few.

Figure 1 : Example of BAR domain protein. Adapted from McMahon & Gallop. 2005. Nature. 438 (7068), 590-596.
We are particularly interested in the role of BAR-domain proteins in endocytic mechanisms, both clathrin-mediated and clathrin-independent. Additionally, we are interested in their ability to sense and then translate plasma membrane deformations into cellular responses.
- Endocytosis:
Unsurprisingly, curvature sensing/inducing BAR domain proteins where shown to play pivotal roles in endocytic processes, where local invaginations of plasma membrane occur. While some BAR domain proteins have been described to play a role during different steps of clathrin-dependent endocytosis (e.g. SNX9, FCHO1/2), others seem to be crucial for clathrin-independent endocytic routes (e.g. GRAF1 and endophilin-A2). However, their exact function in these processes often remains elusive, as it is difficult to know whether they act as curvature sensors or inducers.
Using various approaches including high resolution airyscan confocal as well as proteomics and advanced cell biology techniques, we aim to identify new BAR domain protein/cargo couples and to decipher their underlying mechanism.
Figure 2 : New clathrin-independent cargo (red channel) uptaken in endocytic structures positive for a GFP-tagged BAR domain protein (green channel) at the edge of HeLa cells.
- Mechanotransduction:
Due to their crescent shape, BAR domain proteins could play a role in mechanotransduction by acting as sensors of plasma membrane deformations induced by the cells environment (ex. pressure, sheer stress, stretching). They often contain functionnal domains (ex. RhoGAP, RhoGEF, SH3) that could directly translate mechanical deformations into biochemical signals to later induce a response from the whole cell. Our objective is to understand the relations between BAR domain proteins, curved plasma membrane, actin cytoskeleton and signalling pathways by using several techniques to induce precise nanoscale plasma membrane deformations (ex. nanotopography, AFM) and observing local responses of cells.
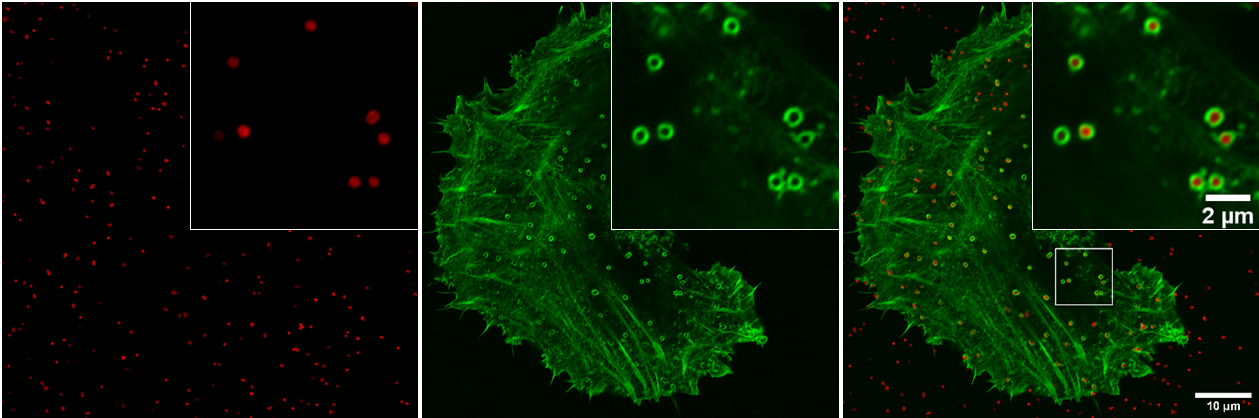
Figure 3 : F-Actin recruitment (green channel) around 500 nm plasma membrane deformations (red channel) in HeLa cells.