Non classé
-
GDT1 transports protons: a new publication in JBC
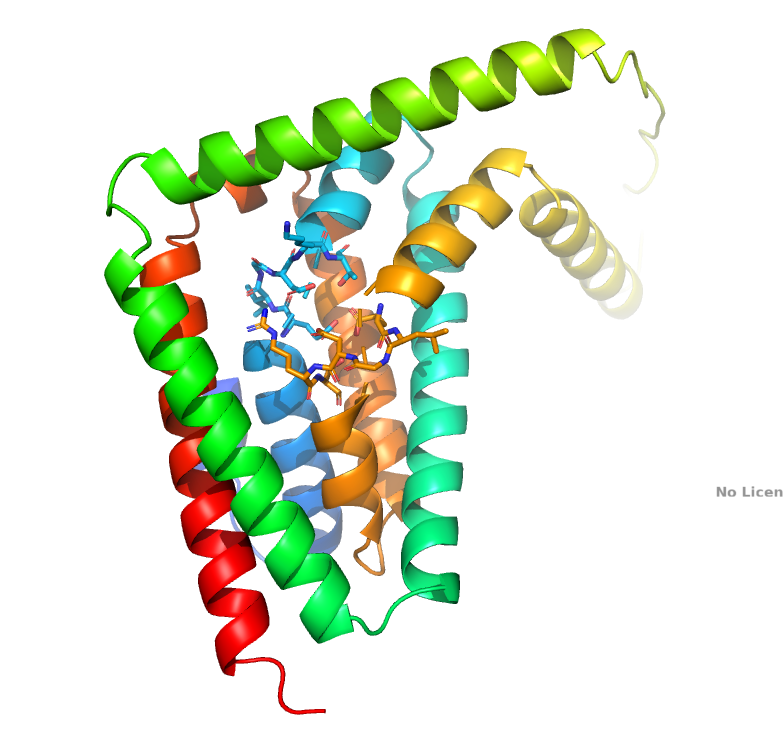
Antoine Deschamps and coworkers have demonstrate that H+ ions are transported in exchange for Ca2+ and Mn2+ cations by the Golgi-localized yeast Gdt1 protein. We performed direct transport measurement across a biological membrane by expressing Gdt1p in Lactococcus lactis bacterial cells and by recording either the extracellular pH or the intracellular pH during the application of Ca2+, Mn2+ or H+ gradients. Besides, in vivo cytosolic and Golgi pH measurements were performed in Saccharomyces cerevisiae with genetically encoded pH probes targeted to those subcellular compartments.
These findings have now been published in JBC.
-
Congratulations to Camille for her PhD thesis
 Bravo Camille ! After François and Benjamin you are the third PhD student of the BAR Team to obtain a PhD. Very nice collaboration between us and UNamur.
Bravo Camille ! After François and Benjamin you are the third PhD student of the BAR Team to obtain a PhD. Very nice collaboration between us and UNamur.Almost at the same time as your paper published in Traffic (see below): N-BAR and F-BAR proteins—endophilin-A3 and PSTPIP1—control clathrin-independent endocytosis of L1CAM
-
Discovery of a new cargo of the Endophilin A3-mediated endocytosis pathway
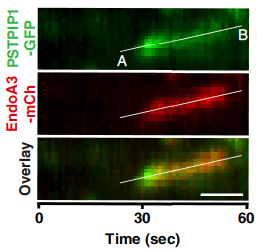
N-BAR and F-BAR proteins—endophilin-A3 and PSTPIP1—control clathrin-independent endocytosis of L1CAM
In the current study, we focus on the endocytosis of L1CAM. This glycoprotein plays a major role in the development of the nervous system, and is involved in cancer development and is associated with metastases and poor prognosis. First, we demonstrated in our cellular context that L1CAM is mainly a clathrin-independent cargo. Second, the mechanism of L1CAM endocytosis is specifically mediated by the N-BAR domain protein endophilin-A3. Third, we discovered PSTPIP1, an F-BAR domain protein, as a novel actor in this endocytic process. This work was mainly conducted by Camille Lemaigre in collaboration with Institut Curie, University of Lund, Galecto and UNamur.
See the publication here
-
Congratulations to Benjamin for his PhD thesis
Beautiful images made by Benjamin during his PhD thesis in collaboration and co-supervision with Christine Dupont, David Alsteens , Henri-François Renard and my lab.
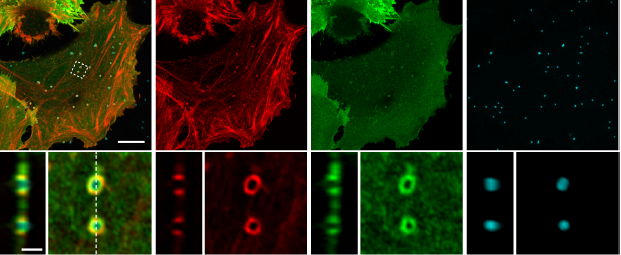
Here, using homemade fluorescent nanostructured cell culture surfaces, Benjamin investigated the role of BAR domain proteins as mechanosensors of plasma membrane geometry. Our data reveal that distinct subsets of BAR proteins bind to plasma membrane deformations in a membrane curvature radius-dependent manner. Interestingly, distinct cellular processes involving BAR domain proteins seem to be primed at these sites of high membrane curvature. We focused on one of them, the local actin cytoskeleton polymerisation. We demonstrated that membrane curvature promotes the formation of dynamic actin structures mediated by the Rho GTPase CDC42, the F-BAR protein CIP4 and the presence of PI(4,5)P2, independently of clathrin.
A preprint version of the paper is visible here
-
Congrats to Andréa and Marie-Odile for their FRIA fellowship
So nice! Marie-Odile and Andréa have obtained a FRIA fellowship to perform a PhD thesis. They will analyse the impact of TMEM165 deficiency on the cell physiology. Let’s go to new and exciting adventures full of fascinating discoveries.
-
Role of actin in EndoA3-mediated endocytosis of CD166
 In mammalian cells, we studied the way a protein called CD166 is removed from the cell surface by endocytosis. In this study, we discovered new molecular components of the mechanism that drives CD166 endocytosis. First, we observed that the skeleton of the cell, composed of actin molecules, appears to assist endophilin-A3 in the formation of the membrane pits that engulf CD166. Secondly, we discovered that the protein Rac1 orchestrates the action of actin in this process. Finally, we observed that the release of vesicles containing CD166 inside the cell is assisted by another component of the cellular skeleton – the microtubules – and motor proteins called kinesins. This work as been performed by François Tyckaert and was co-supervised by Henri-François Renard.
In mammalian cells, we studied the way a protein called CD166 is removed from the cell surface by endocytosis. In this study, we discovered new molecular components of the mechanism that drives CD166 endocytosis. First, we observed that the skeleton of the cell, composed of actin molecules, appears to assist endophilin-A3 in the formation of the membrane pits that engulf CD166. Secondly, we discovered that the protein Rac1 orchestrates the action of actin in this process. Finally, we observed that the release of vesicles containing CD166 inside the cell is assisted by another component of the cellular skeleton – the microtubules – and motor proteins called kinesins. This work as been performed by François Tyckaert and was co-supervised by Henri-François Renard.See the full paper in Journal of Cell Science
Research highlight in JCS
See also a nice interview of François Tyckaert
-
Congratulations to François for his PhD thesis

François Tyckaert is the first PhD student co-supervised by Henri-François Renard and myself.
Using state-of-the-art microscopy and cell biology techniques, François could show that CD166 is a clathrin- and dynamin-independent cargo internalized via a novel endocytic mechanism that involves the BAR domain protein endophilin-A3 and the extracellular lectin galectin-8. Additionally, he could demonstrate that the actin cytoskeleton and its regulatory GTPase Rac1 dynamically associate with CD166-positive endocytic carriers and that their perturbation strongly inhibits the uptake process. He also provided evidence that microtubules and kinesin molecular motors are required to potentiate the endoA3-dependent endocytosis of CD166.
-
Congratulations to Antoine for his PhD thesis

This week Antoine presented us his elegant work on GDT1-mediated proton transport.
In this thesis, a heterologous expression system using Lactococcus lactis was set up for direct H+ transport assay and a Golgi-localized pH sensor was developed for in vivo measurements in S. cerevisiae. Thanks to them, Gdt1p-mediated H+ transport activity was demonstrated. Then, Antoine looked at Gdt1p regulation. He observed that Gdt1p is rapidly downregulated as a response to extracellular Mn2+ addition and that regulation occurs both at transcriptional and post-translational levels.
-
Welcome to Shiqiang as a new PhD student with UNamur
 Shiqiang will study the impact of EndoA3-mediated endocytosis on the immune synapse. An exciting and challenging project in collaboration with Henri-François Renard Team at UNamur, starting in 2020.
Shiqiang will study the impact of EndoA3-mediated endocytosis on the immune synapse. An exciting and challenging project in collaboration with Henri-François Renard Team at UNamur, starting in 2020. -
Guillemette and Quentin have received a FRIA fellowship in 2020
We are very happy to welcome Guillemette and Quentin in our team for PhD thesis.
Guillemette will purify TMEM165 and reconstitute it into proteoliposomes and Quentin will develop new fluorescent dyes to measure calcium and manganese in vivo. Good luck !