Growth Mechanisms for Carbon Nanotubes
Contacts: Jean-Christophe Charlier
Introduction
Nearly fifteen years after their discovery, carbon nanotubes (CNTs) are still attracting much interest for their potential applications, which largely derives from their unusual structural and electronic properties. Since all these properties are directly related to the atomic structure of the tube, it is essential to understand what controls nanotube size, the number of shells, the helicity, and the structure during synthesis. A thorough understanding of the formation mechanisms for these nanotubular carbon systems is crucial to design procedures for controlling the growth conditions to obtain more practical structures, which might be directly available for nanotechnology.
Growth mechanism for multi-wall nanotubes
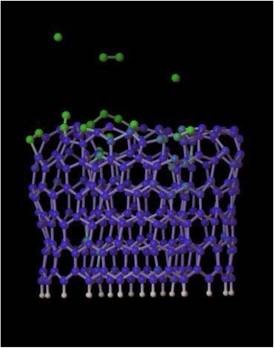
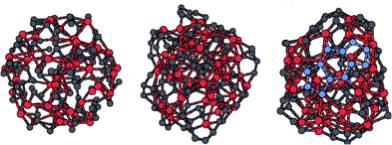
Quantum molecular dynamics simulations were performed to understand the growth process of multi-wall carbon nanotubes. Within such calculations, the topmost atoms (dangling bonds) of the inner and outer edges of a bilayer tube rapidly move towards each other, forming several bonds to bridge the gap between the adjacent edges, thus verifying the assumption that atomic bridges could keep the growing edge of a nanotube open without the need of “spot-weld” adatoms. At ~3000K (a typical experimental growth temperature), the “lip-lip” interactions stabilize the open-ended bilayer structure and inhibit the spontaneous dome closure of the inner tube as observed in analogous simulations of single-shell tubes. These calculations also show that this end geometry is highly active chemically, and easily accommodates incoming carbon clusters, supporting a model of growth by chemisorption from the vapor phase.
Spontaneous closure of SWNTs at ~3000K
Open-ended growth of MWNTs stabilized by “lip-lip” interactions at ~3000K
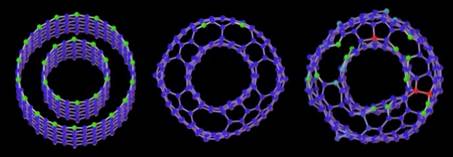
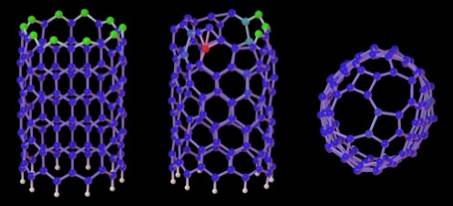
Catalytically assisted growth mechanism for single-wall nanotubes
The catalytic growth of single-wall carbon nanotubes (SWNTs) was investigated using first-principles molecular dynamics simulations. At experimental temperatures (~1500K), even though the open end of SWNTs closes spontaneously into a graphitic dome, the metal-carbon chemical bonds keep breaking and reforming. Such phenomenon provides a direct incorporation process for the necessary additional carbon, and suggests a close-end mechanism for the catalytic growth. The catalytic action of metallic atoms is also found to play a key role in the reconstruction of the nanotube tip after carbon incorporation, by annihilation of defects. The short range action of the metal may explain the relatively narrow diameter observed for SWNTs.
Close-ended catalytic growth of SWNTs at ~1500K
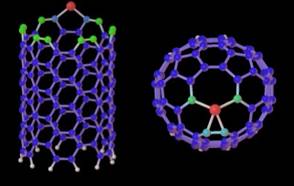
Catalyst assisted growth mechanism for single-wall nanotubes
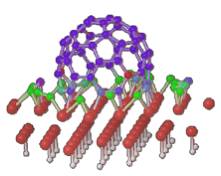
The nucleation and growth of SWNTs within a root growth mechanism (where carbon atoms precipitate from particles larger than the tube diameter) was studied using quantum molecular dynamics simulations. The latters suggest that carbon atoms can be added at the root of a growing tube by a diffusion-segregation process occuring at the surface of the catalytic particle.
First step : segregation of carbon at the nano-particle surface when cooling
from 2000K to 1500K
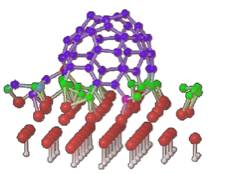
Second step: nucleation and growth of a carbon nanotube germ extruding
from a large catalytic nanoparticle.
Related publications
1) Microscopic growth mechanisms for carbon nanotubes
J.-C. Charlier, A. De Vita, X. Blase, and R. Car
Science 275, 646-649 (1997)
2) Frustration effects and microscopic growth mechanisms for BN nanotubes
X. Blase, A. De Vita, J.-C. Charlier, and R. Car
Physical Review Letters 80, 1666-1669 (1998)
Topics Appl. Phys. 80, 55-81 (2001).
4) Root growth mechanism for single-wall carbon nanotubes
J. Gavillet, A. Loiseau, C. Journet, F. Willaime, F. Ducatselle, and J.-C. Charlier
Physical Review Letters 87, 275504 (2001)
5) Microscopic mechanisms for the catalyst assisted growth of single-wall carbon nanotubes
J. Gavillet, A. Loiseau, F. Ducastelle, S.Thair, P. Bernier, O. Stéphan, J. Thibault,
and J.-C. Charlier, Carbon 40, 1649-1663 (2002).
Main collaborations
Prof. Roberto Car, Princeton University, USA
Dr. Xavier Blase, Université Claude-Bernard, Lyon, FRANCE
Dr. Alessandro De Vita, King’s College, London, UK
Drs. Annick Loiseau and Franćois Ducastelle, ONERA, Chatillon, FRANCE
Main funds
Research Training Network, contract N° HPRN-CT-2000-00128, « COMELCAN : coupled
mechanical and electronic properties of carbon nanotubes based systems », 2000–2003.
Pôle d’Attraction Interuniversitaire P5/01, « Quantum size effects in nanostructured materials » (UCL-FUNDP-KUL-RUCA-UIA) 2002–2006.
European Network of Excellence (N° NMP3-CT-2004-500159) « FAME : Functionalized Advanced Materials and Engineering : Hybrids and Ceramics » 2004–2008.
European Network of Excellence (N° NMP4-CT-2004-500198), « NANOQUANTA : Nanoscale Quantum Simulations for Nanostructures an Advanced Materials » 2004–2008.