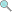4.0 credits
30.0 h + 10.0 h
1q
Teacher(s)
Henrard Séverine ;
Spinewine Anne (coordinator) ;
Language
Français
Main themes
- Evidence-based medicine: basic principles; primary and secondary sources of information: methodology and critical appraisal;
- Methodology of experimental and observational studies relative to the efficacy and safety of medicines, and critical appraisal;
- Methodology of secondary sources of information (systematic reviews, meta-analysis, clinical guidelines), and critical appraisal
- Basic principles in pharmacoeconomy and applications to pharmacotherapy
- Basic principles relative to health insurance, and to economic factors influencing the use of medicines in Belgium and in Europe
Aims
At the end of the course, students should be able:
- To explain the concept of clinical evidence, and the relative strength of different levels of evidence including data from the scientific literature and other sources of information (such as summary of product characteristics, data from the pharmaceutical industry, recommendations from health authorities,
);
- To explain the concept of clinical therapeutic guidelines, and to elaborate on their use in clinical practice ;
- To explain the basic principles of pharmacoeconomy and to explain how it can contribute to make rationale therapeutic choices ;
- To explain the economic and political principles underlying decisions relative to the use of medicines in Belgium and in Europe, and to debate on the role that pharmacists have with that respect.
The contribution of this Teaching Unit to the development and command of the skills and learning outcomes of the programme(s) can be accessed at the end of this sheet, in the section entitled “Programmes/courses offering this Teaching Unit”.
Content
- Evidence-based medicine: basic principles
- Methodology of experimental and observational studies relative to the efficacy and safety of medicines, and critical appraisal;
- Methodology of secondary sources of information (systematic reviews, meta-analysis, clinical guidelines), and critical appraisal
- Pharmacoeconomic studies and their impact on therapeutic choices
- Analysis of the Belgian and European situations with regard to decisions on the use of medicines.
Methods: taught course, clinical vignettes, workshops
Other information
Background knowledge
Pharmacology and pharmacotherapy
Evaluation
Written examination and personal assignment
Faculty or entity<