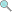5.0 credits
22.5 h + 7.5 h
2q
Teacher(s)
Legrand Catherine ;
Robert Annie ;
Language
Français
Main themes
The following topics will be discussed:
- International guidelines in clinical trials.
- Phase 1: pharmacokinetics and pharmacodynamics.
- Phase 1: dose determination: the continual reassessment method.
- Phases 2 & 3: hypothesis tests in efficacy, superiority or
equivalence trials.
- Phases 2 & 3: power and sample size computation, randomisation and
blinding. Application to sequential trials.
- Phases 2 & 3: cross-over and factorial designs.
- Phase 4: pharmacovigilance. Rare events and risk factors.
- Reporting in clinical trials.
Aims
Objectives The goal of this course is to propose a broad overview of the statistical aspects of phase 1, 2, 3 and 4 clinical trials.
The contribution of this Teaching Unit to the development and command of the skills and learning outcomes of the programme(s) can be accessed at the end of this sheet, in the section entitled “Programmes/courses offering this Teaching Unit”.
Content
The following topics will be discussed:
- International guidelines in clinical trials.
- Phase 1: pharmacokinetics and pharmacodynamics.
- Phase 1: dose determination: the continual reassessment method.
- Phases 2 & 3: hypothesis tests in efficacy, superiority or
equivalence trials.
- Phases 2 & 3: power and sample size computation, randomisation and
blinding. Application to sequential trials.
- Phases 2 & 3: cross-over and factorial designs.
- Phase 4: pharmacovigilance. Rare events and risk factors.
- Reporting in clinical trials.
Other information
References :
Redmond, C. K. and Colton T. (2001), Biostatistics ub Clinical Trials, Wiley.
Fleiss J. (1986), The Design and Analysis of Clinical Experiments. Wiley.
Faculty or entity<
Programmes / formations proposant cette unité d'enseignement (UE)
Program title
Sigle
Credits
Prerequisites
Aims
Master [120] in Biomedical Engineering
Master [60] in Biomedicine
Master [120] in Biomedicine
Master [120] in Statistics: Biostatistics
Master [120] in Mathematics
Master [120] in Statistics: General
Master [120] in Mathematical Engineering