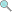4.0 credits
30.0 h + 15.0 h
1q
Teacher(s)
Marko Istvan ;
Riant Olivier ;
Language
Français
Main themes
The thermodynamic and kinetic aspects will be reintroduced and completed by notions of reaction control. The electronic effects will be discussed again and linked to the notions of stabilisation of charges and to acid-basic properties of certain classes of organic functions. The HSAB concept will also be introduced and connected to the concepts of electronegativity and polarization. These principles will be applied to aromatic chemistry and the control of orientation in electrophilic aromatic substitution reactions will be developed and applied to concrete problems of everyday's life (paracetamol, ibuprofen, ..). The chemistry of nitrogen, sulphur and phosphorous will introduce the main classes of functions carrying these heteroatoms as well as their presence in biological molecules (DNA, peptides, ATP,
). A deeper understanding of the mechanisms and the notions of orientation and selectivity will be reached by the study of the main classes of reactions involving the chemistry of these heteroatoms. The interconversions between these functional groups will be completed by the notions of organic intermediates carrying heteroatoms. The knowledge of organic synthesis for the construction of molecules will also be used to illustrate the course in different fields of everyday's life. Introduction to the notion of carbanions. Physico-chemistry and structures. Stabilizing effects. Chemistry of enolates and carbanions will be reintroduced and deepened in the aspects of preparation, reactivity and selectivity. Alkylation reactions, aldol condensations and Michael reaction. Chemistry of unstable organometallics. The organomagnesium, organolithium and organocuprates. Duality between base and nucleophile. Applications in the creation of carbon-carbon bonds. Comparison between different organometallic families.
Aims
In the continuation of the first year organic chemistry course, the emphasis will be placed upon deepening the basic knowledge of chemical reactivity as applied to organic chemistry. The course will be divided in three main complementary parts. In the first part, the notions of control, orientation and electronic effects will be introduced and their application in the chemistry of aromatic compounds exemplified. The second part of the course will describe the fundamentals of heteroatom chemistry. Important classes of biological molecules and biochemical mechanisms will serve as examples to link the theory to the living world. The last part of the course involves the chemistry of carbanions and related organometallic derivatives. The objective is to familiarise the students with the main reactions for carbon-carbon bond formation based upon the use of organometallics and carbanions. The course will also illustrate, through various applications, the aspects of selectivity (regioselectivity, stereoselectivity), essential to learning organic synthesis.
The contribution of this Teaching Unit to the development and command of the skills and learning outcomes of the programme(s) can be accessed at the end of this sheet, in the section entitled “Programmes/courses offering this Teaching Unit”.
Faculty or entity<