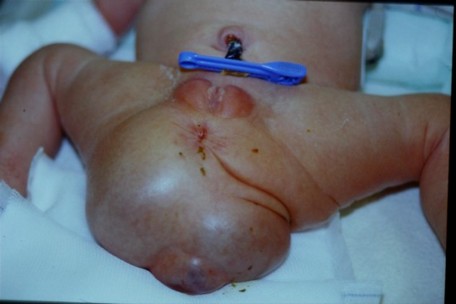
Prevalence: approximately 1 case per 40,000 live births. In 10% of cases, it is associated with sacral abnormalities or a meningomyelocele. This tumor grows from remnants of the primitive crest that should have involuted and disappeared during the caudal regression. Because of its origin, it contains pluripotent cells originating from the 3 cellular embryonnic layers embryo, hence its name of teratoma. The tumor develops at the level of the sacrococcygeal region and can be categorized into 4 main types (Altman classification):
- type I (the most common form): external mass with a small presacral component
- type II: external mass with important intrapelvic component
- type III: barely visible tumor, but large pelvic mass extending into the abdomen
- type IV: presacral mass without external component

Prenatal screening is the rule in type I form and in that case, if the pregnancy is continued, a ultrasound monitoring of the fetus must be repeated regularly to detect any tumor size increase larger than 150 cm3/week but also and above all the emergence of placentomegaly or hydrops indicating a cardiac failure caused by excessive intratumoral blood flow. In these cases, some think a fetal surgery is indicated. The size of the teratoma (> 5 cm diameter) can render a vaginal delivery impossible and therefore require a caesarean section.
The tumor is almost always benign at the time of birth but because of the presence of pluripotent cells, there is a risk of later malignant degeneration. So it is urgent to perform the following exams:
- ultrasound of the tumour to appreciate its content, solid or fluid, and its intrapelvic extension;
- echocardiogram to check the heart anatomy and its hemodynamic tolerance;
- check coagulation for signs of thrombocytopenia or DIC by platelets consumption inside the tumor.
- dosage of α-fetoproteins, usually elevated (and later used as monitoring of postoperative evolution and healing);
- neurological examination.
Apart from an emergency in case of open or necrotic tumor, heart failure or DIC, excision is planned semi-electively in the neonatal period. Surgery must be radical because of the risk of malignancy, although it is low. Some teams recommend, in case of teratoma I or II of large size, to deliver the mother between 27 and 32 weeks of gestation as soon as signs of fetal or maternal decompensation appear before fetal hydrops develops: the procedure (sometimes limited to the debulking of the external part) is performed under EXIT or immediately after birth.
Some teams propose an in utero intervention, in case of rapid growth of the tumor: either hysterotomy for surgery to remove the external tumor, or percutaneous radiofrequency coagulation of the teratoma's feeder vessels under ultrasound control.
In the neonatal period, depending on the type of development and size of the tumor, excision is carried out through an anterior, posterior or combined approach. The long term follow up of patients shows heterogeneous results: very good prognosis with few urinary complications in some series but presence of major urological complications with neurological bladder in 1/3 of cases in other series.
The risk factors for significant transfusion and perioperative mortality are:
- a tumour with non-cystic contents
- a type 1 or 2 tumour
- prematurity (especially < 30 weeks of age of gestation)
- emergency surgical repair
Anesthetic implications: neonatal anesthesia.
in case of anterior surgical approach, the newborn is placed in supine position. In case of posterior surgical approach, it is placed in ventral decubitus with important Trendelenburg position. The limbs are included in the operative field (mobilization of the hips). A roll is placed under the chest to ensure a good chest excursion without abdominal hypertension. If the approach is combined, everything should be organized in order to allow sterile repositioning of the child during the procedure.
Problems to be anticipated:
- a major risk of bleeding either by platelet consumption (DIC) inside the tumor or from surgical origin; moreover, fast and massive transfusion can lead to hyperkalemia
- a risk of cardiac failure linked to the existence of arteriovenous fistulas inside the tumor: some authors propose, in that case, to clip the median sacral artery laparoscopically before the resection of the tumoral mass (notwithstanding the presence of many other feeding vessels)
- invasive blood pressure monitoring and insertion of a venous central line if the mass is important or if there is a significant hemorrhagic risk. If the tumor is small, a peripheral venous access at the upper limb should be sufficient.
References:
- Wilson RD, Hedrick H, Flake AW, Johnson MP, Bebbington MW, Mann S, Rychik J, Liechty K, Adzick NS.
Sacrococcygeal teratomas: prenatal surveillance, growth and pregnancy outcome.
Fetal Diagn Ther 2009; 25:15-20.
- Unal E, Koksal Y, Toy H, Gunel E, Acikgozoglu S.
Neuroblastoma arising from an unresected sacrococcygeal teratoma in a child.
J Pediatr Hematol Oncol 2010; 32:233-5.
- Reinoso-Barbero F, Sepulveda I, Perez-Ferrer A, De Andres A.
Cardiac arrest secondary to hyperkaliema during surgery for a neonatal giant sacrococcygeal teratoma.
Pediatr Anesth 2009; 19:712-4.
- Lukish JR, Powell DM.
Laparoscopic ligation of the median sacral artery before resection of a sacrococcygeal teratoma.
J Pediatr Surg 2004; 39:1288-90.
- Bax NM, van der Zee DC.
The laparoscopic approach to sacrococcygeal teratomas.
Surg Endosc 2004; 18:128-30.
- Roybal JL, Moldenhauer JS, Khalek N, Bebington MW, Johnson MP, Hedrick HL, Adzick NS, Flake AW.
Early delivery as an alternative management strategy for selected high-risk fetal sacroccygeal teratomas.
J Pediatr Surg 2011 ; 46 : 1325-32
- Isserman RS, Nelson O, Tran KM, Cai L, Polansky M, Rosenbloom JM, Goebel TK, Lin EE.
Risk factors for perioperative mortality and transfusion in sacrococcygeal teratoma resections.
Pediatr Anesth 2017 ; 27 : 726-32.
- Au V, Gregory G, Sinskey J.
Intraoperative surfactant administration during neonatal sacrococcygeal teratoma resection.
Pediatr Anesth 2019 ; 29 : 1148-40.
Updated: July 2021