The most common solid tumor in children after brain tumors: estimated incidence of 9.5 cases per million children per year. It is derived from cells of the embryonic neural crest. A mutation in one of the following genes can produce a neuroblastoma: KIF1B gene on 1p36 (NBLST1, MIM 256,700), PHOX2B gene on 4p12 (NBLST2, MIM 613 013), ALK gene on 2p23 (NBLST3, MIM 613 014), a gene on 6p (NBLST4, MIM 613 015), a gene on 2q35 (NBLST5, MIM 613 016), a gene on 1q21 (NBLST 6, MIM 613 017). Other pathologies due to abnormalities during the neural crest migration may be associated with a neuroblastoma it: Hirchsprung disease, Ondine syndrome, type I neurofibromatosis. Depending on the degree of maturation of the tumor cells, one distinguishes: neuroblastoma (little differentiation), ganglioneuroblastoma (intermediate differentiation) or ganglioneuroma (differentiated and benign form).
The mode of presentation is highly variable. The symptomatology depends on the location of the primary tumor and the presence of metastases.
In order of frequency, the primary tumour is:
- abdominal: abdominal mass, sometimes huge in infants, which can lead to respiratory distress and vascular compression;
- thoracic: respiratory problem, Claude Bernard-Horner syndrome; it is located in the posterior mediastinum;
- pelvic: mass, urinary or fecal incontinence;
- cervical: compressive mass.
When the primary tumor is located near the vertebral axis, a tumor extension is often observed into the spinal canal that can cause pain or signs of spinal compression (walking disorders, acute paraplegia).
Metastases can be:
- medullary: anemia, leukopenia, thrombopenia;
- hepatic: liver failure by invasion;
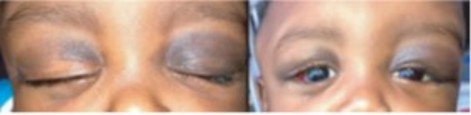
- retro-orbital: exophthalmia associated with periorbital bruising (Hutchinson's sign); this location is pathognomonic but often initially attributed by error to a trauma.
- mandibular: painful swelling of the jaw
The child may also present with general signs such as:
- systemic hypertension, due to compression of the renal vessels by the mass or tumor secretion of catecholamines (sometimes associated with profuse sweating)
- a watery diarrhea, due to the secretion of VIP (Vasoactive Intestinal Peptide) by the tumor, and causing dehydration with hypokaliemia, hypochlorhydria and hypercalcemia;
- neurological signs: Kinsbourne syndrome or opso-myoclonia (an association of incessant and intense myoclonia, making coordinated movements impossible, and opsoclonias, which are anarchic, asymmetrical and multidirectional movements of the eyeballs) (see this term)
Tumors secreting VIP or causing opso-myoclonia are histologically more mature and have a better prognosis. In general, infants have an L1 or MS stage, while children over one year of age are more likely to have an L2 or M stage.
The initial extension assessment is essential to adapt the treatment.
This includes, in addition to a hematological check-up and a bone marrow aspiration/biopsy:
- measurement of urinary catecholamines: catecholamines or their metabolites (homovanillic or vanillylmandelic acid or metanephrine) are found in over 90 % of cases; the presence of vanillylmandelic acid and/or metanephrine (metabolites of norepinephrine metabolism) carries a much higher risk of intraoperative hypertension than the presence of homovanillic acid (a metabolite of dopamine)
- serum markers: neuron-specific enolase, LDH;
- MIBG scan (meta-iodobenzylguanidine marked with iodine 123) to confirm the diagnosis, detect metastases and ensure the follow-up after treatment.
A biopsy of the tumor is necessary to know the histological aspect (degree of cell maturation), the DNA content of tumor cells and the amplification of MYCN (oncogene located on 2p24.3 the expression of which stimulates tumor growth).
A new clinical classification has been established based on the initial tumoral extension. It estimates the vital risk and defines a therapeutic strategy. The clinical elements are weighted according to the results of the biological results knowing that low cell differentiation, NMYC amplification, or low DNA content are pejorative risk factors.
|
grade INRGSS International Neuroblastoma Risk Group Staging System |
grade INSS International Neuroblastoma Staging System |
definition |
therapeutic
|
|
L1 |
1 et 2 |
Localized tumor that does not invade vital structures (imagery) and is limited to one body compartment |
surgical resection |
|
L2 |
3 |
Locoregional tumor with one or more risk factors for local invasion at imagery |
Difficult surgical resection due to the invasion of vital structures |
|
M |
4 |
tumor with remote metastases (except MS): lymph nodes, bone marrow, liver, other organs |
Aggressive preoperative medical treatment is required |
|
MS |
4S |
Tumor in an infant less than 18 months of age and with metastases limited to the liver, skin and/or bone marrow |
Surgery is not necessary unless life-threatening complications occur |
International Neuroblastoma Clinical Classification System
The treatment strategy is established on a case-by-case basis:
- in case of low risk (stage L1 without NMYC amplification), surgery alone is necessary;
- in cases of high risk (stage L2 with amplification of NMYC or adverse histology, stage M), chemotherapy (cyclophosphamide, vincristine, anthracycline) is administered before surgical resection which is performed when the tumoral volume has decreased; surgery is followed by other chemotherapies and, ideally, autologous bone marrow transplantation and, if necessary, local radiotherapy.
The MS stage (sometimes called Pepper syndrome) is special because it does not require, in principle, any treatment, unless it has an amplification of NMYC or an unfavorable histology, since the tumor regresses spontaneously. However, if the tumor is very large and life-threatening (respiratory distress, liver failure), chemotherapy is administered to accelerate tumor regression.
New therapeutic modalities (MIBG marked with iodine 131, retinoic acid etc.) are being evaluated.
Anesthetic implications:
- preoperatively:
- echocardiography to assess the myocardial function that can be impaired by chemotherapy (anthracyclines) as well as a cardiomyopathy induced by a chronic excess of circulating catecholamines
- measure of urinary catecholamines
- hematological check-up
If the tumor secretes catecholamines, a preoperative antihypertensive therapy should be started to progressively compensate for the vasoconstriction-induced hypovolemia and prevent or improve a catecholamine-induced cardiomyopathy. The most widely used treatment consist in a calcium inhibitor (nifedipine or nicardipine, for example) or labetalol.
- perioperatively:
- during tumor diagnosis and evaluation procedures: risk of high blood pressure during tracheal intubation or palpation of the tumor, and risk of hypotension due to a supine hypotension syndrome caused by the tumor mass.
- risk of delayed gastric emptying due to abdominal compression
- if the tumor secretes catecholamines: management similar to that of a pheochromocytoma (see this term)
- if the tumor does not secrete catecholamines and even if the child did not have preoperative high blood pressure, it is common for the manipulation of the tumor to cause hypertensive crises that are treated by deepening anesthesia or administering a short-acting vasodilator (e.g. nicardipine, magnesium sulphate, nitroprusside)
- it is common for the exeresis of the tumor to be followed by significant low blood pressure due either to a delay in filling masked by vasoconstriction or to the sudden interruption of the production of vasoactive agents: this hypotension usually responds to vascular filling but sometimes a vasoconstrictor is given temporarily
- invasive blood pressure monitoring, central venous pressure, urinary catheter
- in case of abdominal tumor, it is better to insert the IV catheters in the upper limbs in order to be able to ensure effective vascular filling in case of IVC cross-clamping.
- in the absence of hemostatic disorders, it is useful to associate an epidural block with general anesthesia. However, the risk-benefit ratio of the technique must be individualized because the presence of an abdominal tumor leads to an increased risk
of venous effraction (collateral circulation through the epidural venous network) and major hypotension) when the increased sympathetic tone that counterbalances the compression of the inferior vena cava is abolished by the sympathetic bloc (supine hypotensive syndrome as during the last trimester of pregnancy). In some cases, it is wise to perform the epidural injection of LA only when the tumor has been removed. It is also important to ensure that there are no vertebral metastases or tumor invasion of the vertebral spinal canal
- in case of thoraco-abdominal tumor encompassing the thoracic aorta with a risk of involvement to the Adamkiewicz artery during surgical dissection, some teams place preoperative lumbar drainage of the CSF to reduce the risk of spinal cord ischemia
- if the abdominal tumor is small and well delineated (stage 1 or 2), it is possible to perform surgery under laparoscopy. In the case of a stage 3 or 4 abdominal tumor, the procedure can be long and delicate, and sometimes more mutilating than expected: sometimes a nephrectomy, pancreatectomy or partial colectomy is required.
- given the significant fluid losses, it may be helpful to admit the child in the intensive care unit for post-operative care. In case of abdominal tumors close to large vessels, it is not uncommon for the postoperative period to become complicated by lymphorrhea or even chylous ascites.
- in case of mandibular metastasis: risk of difficult intubation
- the child may also have severe intraoperative hyperthermia that appears to be related to a hypermetabolic crisis secondary to an acute catecholaminergic discharge, that can be difficult to distinguish from a malignant hyperthermia crisis
References :
- Joseph T, Olivier B, Magnier S, Brugières L, Casasoprana A.
Myocardiopathie dilatée aux catécholamines dans le neuroblastome.
Arch Pédiatr 1997 ; 4 : 32-5.
- Goldsby RE, Matthay KK.
Neuroblastoma : evolving therapies for a disease with many faces.
Pediatric Drugs 2004; 6: 107-22.
- Seefelder C, Sparks JW, Chirnomas D, Diller L, Shamberger RC.
Perioperative management of a child with severe hypertension from a catecholamine secreting neuroblastoma.
Pediatr Anesth 2005; 15: 606-10.
- Tobias JD.
Preoperative blood pressure management of children with catecholamine-secreting tumors : time for a change.
Pediatr Anesth 2005; 15: 537-40.
- Mayhew JF.
Intraoperative hyperthermia in a child with neuroblastoma.
Pediatr Anesth 2006; 16: 890-1.
- Hernandez MR, Shamberger RC, Seefelder C.
Catecholamine-secreting neuroblastoma in a 4-month-old infant: perioperative management.
J Clin Anesth 2009; 21: 54-6.
- Dhir S, Wheeler K.
Neonatal neuroblastoma.
Early Human Dev 2010; 86:601-5.
- Thillaivasan A, Arul GS, Thies K-C.
Vasopressin for the treatment of catecholamine-resistant hypotension during pheochromocytoma repair in a 6-year-old child.
Eur J Anaesthesiol 2010; 27: 991-2.
- Wang C, Xin W, Ji Y.
Hyperthermia in a pediatric patient with neuroblastoma during anesthesia: a case report.
BMC Surg 2021; 21:112 doi: 10.1186/s12893-021-01124-3
- Fu W, Church J, Garton H, Geiger J, Newman E.
Pre-operative lumbar drain placement: a technique for minimizing ischemic spinal cord injury during neuroblastoma resection.
J Pediatr Surg 2022; 57:1443-5
- do Amaral-Silva GK, Leite AA. Mariz BALA et al.
Metastatic neuroblastoma to the mandible of children: report of two cases and critical review of the literature.
Head and Neck Pathol 2021 ; 15 :757-68
- Gao S, Dong Y.
Acute left heart failure with pulmonary edema during resection of pediatric neuroblastoma: case report.
Brazil J Anesthesiol 2022;72:156-8
- Liu J, Zurakowski D, Weldon C, Umaretiya P, Holzman R, LinY-C.
Perioperative hypertension and anesthetic management in patients undergoing resection of neuroblastoma.
Pediatr Anesth 2023;33: 577- 82.
- Larkins M, Iasiello J,Travia K, Pasli M,Cai S, Hutton A.
Implementation of the American Society of Anesthesiologists 2022 paediatric guidelines in a child with mandibular metastasis.
Anaesthesia Reports 2024; 12, e12274
Updated: February 2024