(Adrenogenital syndrome)
Group of 7 diseases with recessive autosomal transmission of mutations of genes coding for enzymes involved in the biosynthesis of cortisol . Most of the patients are carriers of heterozygous mutations (a different mutation on each allele of the gene ), which explains the variability of the clinical presentations resulting of the action of the most functional enzyme. The most frequent form (90 % of cases; prevalence 1/14,000) is the deficiency in 21-hydroxylase (mutation of the CYP21A2 (6p21.3)) responsible for the transformation of progesterone in desoxycorticosterone.
The classic form of this pathology is clinically expressed, from birth in most cases, mainly under 2 forms:
- a form resulting in signs of virilization (25% of cases), easier to recognize in the girl than in the boy;
- a form resulting in a salt wasting syndrome and compromising the vital prognosis in the absence of diagnosis and early treatment.
In the so-called nonclassic forms (estimated prevalence between 1/200 to 1/1000), the deficit is incomplete and the enzyme abnormality does not appear clinically until the time of puberty by signs of virilization (they are still more easily identifiable in girl than in boy). Some forms are paucisymptomatic but may be the cause of some forms of infertility.
Other constitutional enzyme deficiencies that can cause congenital adrenal hyperplasia are listed in both tables. The StAR deficiency (an acronym for Steroidogenic Acute Regulatory protein) is responsible for a severe clinical form known as congenital lipoid hyperplasia of the adrenals.
In about 10 % of the cases, the classic form of congenital adrenal hyperplasia is associated with an hypermobile form of the Ehlers-Danlos syndrome: the CAH-X syndrome, a continous genes syndrome by deletion of the CYP21A2 and TNXM (coding for the thenascine X (6p21)) (see this term)
.jpg)
|
defect |
21-hydroxylase |
11â-hydroxylase |
17á-hydroxylase or 17,20-lyase |
3â-hydroxy steroid déshydrogenase |
P450 oxydo-
|
Lipoid
|
Cholesterol side chain cleavage enzymz |
|
gene |
CYP21A2 |
CYP11B1 |
CYP17A1 |
HSD3B2 |
POR |
StAR |
CYP11A1 |
|
locus |
6p21.3 |
8q24.3 |
10q24.32 |
1p12 |
7q11.23 |
8p 11.23 |
15q24.1 |
|
MIM |
|||||||
|
incidence |
1/10.000 |
1/100.000 (+ Moroccan Jews) |
1/50.000
|
very rare |
very rare |
very rare (mostly Japan, Corea, Palestine) |
extremely rare (eastern Turkey) |
|
salt wasting |
yes if classic |
no |
no |
yes |
no |
yes if classic |
yes if classic |
|
hypertension |
no |
yes if classic |
yes |
no |
yes |
no |
no |
|
virilization |
yes if classic |
yes if classic |
no |
no |
no |
no |
no |
|
sex steroid hormone deficiency |
no |
no |
yes |
yes if classic |
yes |
yes if classic |
yes if classic |
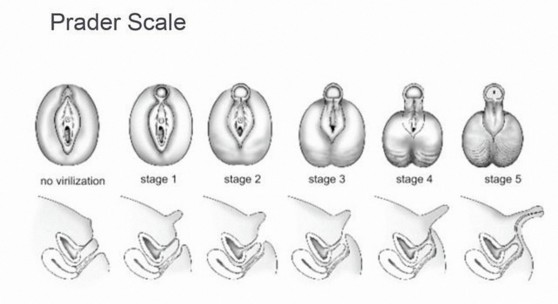
The 5 stages of virilization of female external genitalia according to Prader
Congenital adrenal hyperplasia is systematically detected at 3 days of age by the radioimmunoassay test of 17 OH-progesterone. This test allows an early diagnosis before the onset of the salt wasting syndrome which usually occurs around 2-3 weeks of life.
The treatment depends on bio-assays. It is based on the compensation of glucocorticoid deficiency by hydrocortisone and, if necessary, the deficiency in aldosterone (to reduce plasma concentrations of angiotensin II). It is also important to administer aromatase inhibitors (to slow down skeletal maturation) and anti-androgen (cyproterone, flutamide) to lower androgen levels and avoid virilization in young girls. In case of 21-hydroxylase deficiency, recent studies have shown the efficacy of the oral administration (2x/day) of crinecerfont, a corticotropic releasing factor type 1 receptor antagonist, in reducing adrostenedione blood levels and thus their side effects.
Anesthetic implications:
- in the neonatal period, in presence of a salt wasting syndrome with severe dehydration before that the diagnosis of the disease is made;
- determination of blood electrolytes, blood glucose and 17-OH-progesterone, renin, cortisol and aldosterone because the hypothesis of congenital hyperplasia should be systematically evoked in the face of unexpected dehydration in the neonatal period, especially in a girl with signs of virilization;
- hydroelectrolytic rehydration with a bolus of 20 mL/kg of 0.9 % NaCl in 1 hour and repeated as needed until a satisfactory hemodynamic state is restored. Children with 21-hydroxylase deficiency are usually hyperkalemic: do not provide them with K+, and therefore avoid Ringer lactate; only those with 11-â-hydroxylase or 17-α-hydroxylase deficiency may be hypokalemic and require K+ supplementation: wait for ionogram results before considering administering a solution containing K+ ;
- supply glucose if the child is hypoglycemic, and afterwards in the basal infusion which is to be continued after the restoration of euvolemia.
- determination of corticosteroids and mineralocorticoids in case of deficiency in 21-hydroxylase:
hydrocortisone p os: 10-15 mg/m2/day in 3 divided doses
fludrocortisone p os: 0.05 to 0.2 mg/day in 2 divided doses
supplements of NaCl 1 to 2 g/day (in childhood)
These starting doses are adapted on the basis of biological controls and growth of the child (more difficult balance during puberty): adult,
15 to 25 mg/day in 2 or 3 doses
- in case of fever, gastroenteritis with dehydration, major trauma: IV intake of glucose and electrolytes and of the initial dose hydrocortisone
25 mg in small children
50 mg in children of school age
100 mg in adults
Subsequent doses are calculated on basis of 3 to 4 times the usual daily dose divided into 4 doses per day until return to a normal situation.
- during anesthesia , either for the surgical treatment of the virilization of the genitalia of a girl, for the treatment of intercurrent surgical pathology (in both sexes):
Recommendations of the Haute Autorité de santé (France), April 2011
* in case of acute decompensation (excessive fatigue, nausea, abdominal pain, hypotension, pallor, sweating): IV hydrocortisone 2 mg/kg every 4-6 hours and fluid and electrolyte resuscitation
* in case of major surgery :
a) hospitalization the day before the intervention and start IV administration of a glucose-containing hydroelectrolytic solution to meet basic needs; the usual corticosteroid dose is doubled on the evening of the intervention;
b) a 'stress' dose of hydrocortisone is given at induction of anesthesia (2 mg/kg or 50 - 100 mg/m2), and repeated every 4-6 hours until the resumption of food and the usual treatment;
c) there is no injectable form of mineralocorticoid; for patients with aldosterone deficiency, it is useful to take an oral dose of fludrocortisone (50 to 200 µg) 2-3 hours prior to the intervention.
* in case of minor surgery or examination requiring a period of fasting: a 'stress' dose of hydrocortisone is given at induction of anesthesia (2 mg/kg or 50 - 100 mg/m2), and repeated every 4-6 hours until the resumption of food.
N.B.: the principle of doubling the dose of corticosteroids on the eve of surgery is not based on any evidence and is recommended neither by The Endocrine Society, nor the APAGBI, nor the Royal College of Physicians. It is probably better to administer the usual treatment on the the morning of surgery and to administer a small dose of hydrocortisone (2 mg/kg) at the induction of anesthesia, then:
* major surgery: continuous infusion of 25 mg/day if < 10 kg
50 mg/day if 11-20 kg
100 mg/day if > 20 kg and prepubertal
150 mg/day si > 20kg and puberty is
present
or 2 mg/kg/4h
* minor surgery : double the oral dosis when feeding is allowed, during 24hr
Equivalence table of the steroids
|
|
Equivalent Dose (mg) |
Relative power |
|
hydrocortisone |
20 |
1 |
|
prednisolone |
5 |
4 |
|
methylprednisolone |
4 |
5 |
|
dexamethasone |
0,75 |
25 |
References:
- White PC, Speiser PH.
Congenital adrenal hyperplasia due to 21-hydroxylase deficiency
.
Endocrine Reviews 2000; 21:245-291.
- Lambert SM, Vilain EJ, Kolon TF.
A practical approach to ambiguous genitalia in the newborn period.
Urol Clin North Am 2010; 37:195-205.
- Ruppen W, Hagenbuch N, Jöhr M, Christen P.
Cardiac arrest in an infant with congenital adrenal hyperplasia.
Acta Anaesthesiol Scand 2003; 47: 104-5
- Speiser PW, Azziz R, Baskin LS, Ghizzoni L et al.
Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency : an endocrine society clinical practice guideline.
J Clin Endocrinol Metab 2010; 95: 4133-60.
- El-Maouche D, Arlt W, Merke DP.
Congenital adrenal hyperplasia.
Lancet 2017; 390: 2194-2210
- Merke DP, Auchus RJ.
Congenital adrenal hyperplasia due to 21-hydroxylase deficiency.
NEJM 2020; 383: 1248-61
- Woodcock T, Barker P, Daniel S, Fletcher S et al.
Guidelines for the management of glucocorticoids during the peri-operative period for patients with adrenal insufficiency.
Anaesthesia 2020;75:654-73
- Heath C, Siafarikas A, Sommerfield A, von Ungern-Sternberg BS.
Peri-operative steroid management in the paediatric population.
Acta Anaesth Scand 2021; 65:1187-94.
- Sarafoglou K, Kim MS, Lodish M, Felner EI, Martinerie L, Nokoff NJ, Clemente N, Fechner PI et al for the CAHtalyst Pediatric Trial Investigators.
Phase 3 trial of Crinecerfont in pediatric Congenital Adrenal Hyperplasia.
NEJM 2024 ; DOI: 10.1056/NEJMoa2404655
Updated: June 2024