Group of hereditary diseases characterized by iron overload.
Their common characteristics are:
1) an increased saturation coefficient of transferrin as first biological anomaly
2) a progressive involvement of the liver, endocrine glands, joints (arthritis) and heart (except type 4A),
3) a good response to phlebotomies
(except type 4A)
4) an absence of anemia (except after the bloodletting)
Clinical signs: skin hyperpigmentation (tanned color), hepatic fibrosis followed by cirrhosis (with risk of carcinoma), diabetes mellitus, hypogonadism, cardiomyopathy.
There are several types of hereditary hemochromatosis:
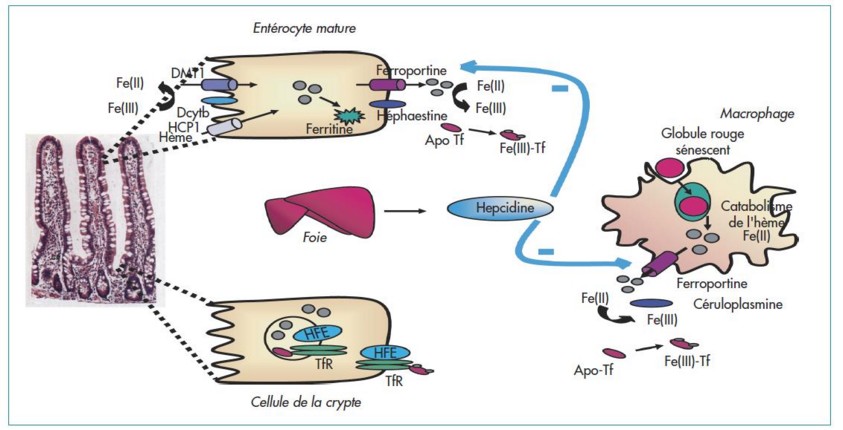
- type 1 or classic [MIM 235 200], the most common: autosomal recessive transmission with variable penetrance of a mutation in the HFE gene (6p21.3) coding for the HFE protein. The HFE protein combines with the receptor 1 transferrin and modulates the amount of iron that enters into the cells; onset between 40 and 50 years of age
- type 2A [MIM 602 390]; autosomal recessive transmission of a mutation of the HJV gene (1q21) coding for hemojuvenil; onset between 10 and 30 years of age; high mortality due to heart failure or rhythm disorders
- type 2B [MIM 613 313]: autosomal recessive transmission of a mutation of the HAMP gene (19q13.1) coding for hepcidin. Hepcidin is synthesized in response to inflammatory condition and iron overload and binds to ferroportin causing its degradation; onset between 10 and 30 years of age
- type 3 [MIM 604 250], autosomal recessive transmission of a mutation of the TFR2 gene (7q22.1) coding for the receptor 2 of transferrin allowing the internalization of the iron-transferrin complex; onset between 20 and 30 years of age
- type 4 or ferroportin disease [MIM 606 069]; prevalence: < 1.106; autosomal dominant transmission of a mutation of the SLC40A1 gene (2q32.2) coding for ferroportin, which is involved in the excretion of the iron out of the enterocytes and macrophages.
The mutation causes:
* either a loss of function (A form) and an accumulation of iron in the reticulo-endothelial cells of the liver and spleen; the saturation coefficient of transferrin is normal or low, and the serum ferritin level is high; it generally remains asymptomatic but hepatic fibrosis can appear
* either a gain of function with resistance to hepcidin (B form): the symptomatology is then similar to the 1, 2 and 3 types. Onset between 40 and 50 years of age.
- type 5 [MIM 615 517] autosomal dominant transmission of a mutation of the FTH1 gene (11q13.12)
- African hemochromatosis (also known as Bantu siderosis) [MIM 601 195]; association of a possible congenital anomaly of the recycling of erythrocytic iron (HFEA gene) with an excessive dietary intake of iron (beer); accumulation of iron in the hepatocytes and the Küpffer cells.
Other congenital causes of accumulation of iron in the tissues are:
· aceruloplasminemia (see this term)
· microcytic anemia with liver iron overload: rare, due
- either a mutation in the gene SLC11A2 (12q13.12) [MIM 206 100]
- either a mutation in the gene STEAP3 (2q14) [MIM 615 234] or severe congenital hypochromic anemia with ringed sideroblasts they involve means the DMT1 carrier protein insuring the transport of ion from the intestinal lumen into the cytoplasm of the enterocytes of the duodenum.
Causes of secondary or acquired hemochromatosis are: chronic blood transfusions, excess of dietary or parenteral iron.
Differential diagnosis:
- iron overload: post-transfusion (thalassemia, sickle cell disease, hematological diseases)
- hyperferritinemia: alcoholism, inflammatory diseases
Treatment: repeated phlebotomies which are effective if they are started before the onset of tissue damage.
Anesthetic Implications:
a ferritin level > 1,000 µg/l is the biological sign of the severity of iron overload: in these cases, ECG (conduction disorders ?), echocardiography (congestive cardiomyopathy ?), blood sugar (diabetes mellitus), liver function (fibrosis - cirrhosis - portal hypertension ?)
References :
- Shander A, Berth U, Betta J, Javidroozi M.
Iron overload and toxicity: implications for anesthesiologists.
J Clin Anesth 2012; 24: 419-25
- Coppin H, Roth M-P.
Hémochromatoses héréditaires: les molécules en jeu et leur implication dans la régulation de l’homéostasie du fer.
Hépato-Gastro 2005; 12: 281-8
Updated: April 2019