Different chronic liver diseases with cirrhosis can cause, in some patients, including young children (prevalence: 2-8 %), a modification of the pulmonary vasculature which causes an abnormal respiratory exchange known as hepato-pulmonary syndrome. The pathophysiology of this anomaly combines an anomalous pulmonary vasodilation associated with an impaired distribution of ventilation and perfusion. The problem can also occur in case of portosystemic congenital shunts or following a Fontan procedure due to high venous hepatic pressure.
The diagnosis of hepato-pulmonary syndrome is based on the presence of the following triad:
- liver failure, sometimes moderate, because there is no correlation between the severity of hypoxemia and liver failure, associated with portal hypertension;
- moderate to severe hypoxemia (PaO2 at room air < 70 mmHg or SpO2 < 94 %) with orthodeoxia (hypoxemia that worsens in standing position : SpO2 decreases more than 5 % when standing from the supine position); digital clubbing is frequent
- a pulmonary vasodilation responding weakly to the administration of 100 O2.
In case of hepatic insufficiency and/or portal hypertension, the decrease in the hepatic degradation of vasodilating substances (glucagon, NO...) causes vasodilation at the level of the pulmonary microvascularisation and, consequently, an increase in alveolar-arterial gradient corrected for age (with or without hypoxemia).
Three physiopathological mechanisms, often combined, are possible:
1) presence of a true shunt, due to intrapulmonary or porto-pulmonary arteriovenous anastomoses: this situation is rare in childhood in the absence of portal atresia; even with administration of 100 % O2, PaO2 does not reach 150 mmHg; it is defined as hepatopulmonary type II syndrome, which can be partially corrected by embolisation of the large anastomoses
2) increase in the physiological intrapulmonary shunt due to a poor distribution of ventilation and perfusion by inhibition of hypoxic vasoconstriction;
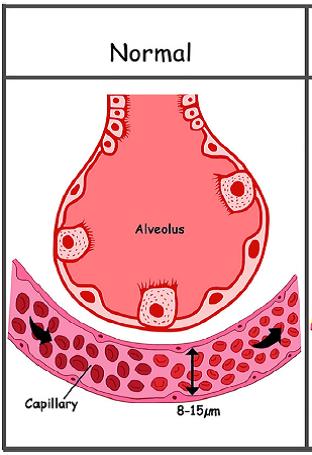
3) decrease in the diffusion of O2 at the level of the dilated pulmonary capillaries (normal diameter 8-15 µm vs 50 - 80 µ in case of hepato-pulmonary syndrome); in case of anomalies 2) and 3), it is defined as hepatopulmonary type I syndrome
In both last situations, the response to the administration of 100 % O2 results in a PaO2 increase > 300 mmHg . Orthodeoxia (decrease of > 5% of SPO2 from the supine to the standing position) is explained by the fact that vasodilation predominates at the lung bases: the shunt is therefore increased in standing position.
Severity: moderate if PaO2 60-79 mmHg
severe if PaO2 50-59 mmHg
very severe if PaO2 ≤ 50 mmHg

Other possible causes of hypoxemia are numerous in case of liver failure: pleural effusion, important ascites, bronchopulmonary infection, alveolar hypoventilation, pulmonary hypertension, combined cyanotic heart disease .
In addition to the response to 100% O2 administration, the following tests may be performed to confirm the presence of hepato-pulmonary syndrome:
- nuclear medicine: measuring the ratio R between the cerebral and the pulmonary uptake 20 min after the injection of microaggregates of Tec99-labelled albumin:
- if R < 1: absence of shunt
- if R is between 1 and 2: questionable result or subclinical hepato-pulmonary syndrome,
- if R is between 4% and 20%: there is a moderate shunt; minor hepatopulmonary syndrome
- if R is between 21% and 40%: moderate hepatopulmonary syndrome
- if R > 40%: severe hepatopulmonary syndrome
- cardiac echography with IV injection of stirred NaCl 0.9% (which produces small air bubbles with a contrast effect): the ultrasound probe is placed so as to see the two atria. The bubbles are seen arriving in the RA and one can look for their passage in the LA:
- absence of microbubbles in the LA: normal picture, there is no shunt.
- if microbubbles are spotted in the LA after a very short time (<3 cycles), there is an intrapulmonary shunt;
- if microbubbles are spotted in the LA 3-6 beats after their appearance in the RA, there is a hepatopulmonary syndrome.
This test is difficult to perform in tachycardic children but is more sensitive than nuclear medicine because it detects the hepatopulmonary syndrome at an early stage, before hypoxemia develops. Some teams recommend for these examinations in children with less than 94% SpO2 at room air.
Medical treatment is not very effective: various molecules have been tried such as almitrine bismesylate or octeotride but their effectiveness is limited. A transjugular porto-systemic shunt (TIPS) is sometimes placed temporarily.The only curative treatment is liver transplantation but the pathology restarts in case of graft rejection or portal thrombosis.
N.B.: a particular form of type II hepatopulmonary syndrome is not linked to an hepatic disease. It is the apparition of pulmonary arteriovenous fistulae after a Glenn's bidirectional cavopulmonary shunt. Those fistulae disappear progressively when thehepatic venous blood flows again through the pulmonary arteries (Fontan's circulation).
Anesthetic implications:
anesthetic management for a child suffering from a hepatopulmonary syndrome involves:
- the eviction of the N2O and the titration of FiO2 so as to obtain a SpO2 between 95 and 98%;
- avoiding ventilation in pure O2 (which promotes atelectasis);
- maintenance of a slight PEEP (Positive End-Expiratory Pressure);
- the accurate replacement of fluid losses to prevent hypovolemia and anemia but also any vascular overload (risk of pulmonary edema).
- in case of systemic hypotension, titrated norepinepherine is the best option; 'vasodilating' drugs as dobutamine or nitroglycerin are ineffective;
- rapid weaning from the ventilator at the end of intervention.
- traps to avoid:
1) a refractory hypoxemia may arisein the first hours after a liver transplantation (SpO2 < 85 % even with FIO2 = 1 and use of PEEP).It is probably due to the vasoconstriction of pulmonary vessels in the presence of hepatic mediators.
To be tried successively: Trendelenbourg position, NO or epoprostenol administration, methylene blue, embolization of the still dilated arteries, ECMO. Significant mortality (45 %).
2) the pulmonary vasodilation may mask mild portopulmonary hypertension (see this term) that will become clinically evident only after the transplantation.
References :
- Al-Hussaini A,Taylor RM, Samyn M, Bansal S, Heaton N, Rela M, Mieli-Vergani G, Dhawan A.
Long-term outcome and management of hepatopulmonary syndrome in children.
Pediatr Transplant 2010; 14:276-82. - Mazzeo AT, Lucanto T, Santamaria LB.
Hepatopulmonary syndrome: a concern for the anesthetist ? Preoperative evaluation of hypoxemic patients with liver disease.
Acta Anaesthesiol Scand 2004; 48:178-86. - Van Obbergh LJ, Carlier M, De Kock M, Otte JB, Moulin D, Veyckemans F.
Hepatopulmonary syndrome and liver transplantation: a review of the perioperative management of seven paediatric cases.
Paediatr Anaesth 1998; 8:59-64.
- Karrer FM, Wallace BJ, Estrada AE.
Late complications of biliary atresia: hepatopulmonary syndrome and portopulmonary hypertension.
Pediatr Surg Int 2017; 33: 1335-40. - Lee WS, Wong SY, Ivy D, Sokol RJ.
Hepatopulmonary syndrome and portopulmonary hypertension in children: recent advances in diagnosis and management.
J Pediatr 2018; 196; e14-21. - Sonavane AD, Badge A, Raur V, Marar S, Sawant A et al.
Therapeutic coil embolization of dominant shunt in hepatopulmonary syndrome enhances post-liver transplant respiratory recovery.
Pediatr Transplant 2020; 24: e 13729 - Gendera K, Eicken A, Ewert P.
Spontaneous closure of arterio-venous pulmonary fistulas by redirection of hepatic venous blood 9 years after Glenn anastomosis in a 12-year-old girl.
Cardiology in the Young 2019; 29:1287-9 - Fauconnet P, Klopfenstein CE, Schiffer E.
Hepatopulmonary syndrome: the anaesthetic considerations.
Eur J Anaesthesiol 2013; 30 :721-30. - Nayyar D, Man HSJ, Granton J, Lilly JB, Gupta S.
Proposed management algorithm for severe hypoxemia after liver transplantation in the hepatopulmonary syndrome.
Am J Transplantation 2015; 15: 903–13
Updated: July 2022