Prevalence: 1/5,000 to 1/15,000 live births with a clear trend for an increase over the last decade: from 3.92 to 6,17 per 10,000 births in Texas from 1999 to 2008. There is a slight male predominance. The malformation is more common in young women with high-risk behaviours (smoking, recreational drugs, genitourinary infections, herpes) and with a low number of pregnancies. It is possible that a mutation of one of the NOS3, ADD1, ICAM1, ICAM4 or ICAM5 genes exacerbates the impact of environmental factors on fetal development.
The anomaly consists in the absence of closure of the abdominal wall that presents as a longitudinal slot located laterally, usually in the rectus abdominus muscle on the right side, at the level of the base of the umbilical cord, which is not involved in the defect. As a result, a more or less important part of the midgut remains extra-abdominal and bathes in utero in the amniotic fluid without direct tissue coverage. At birth, the viscera (small bowel, sometimes liver and spleen) are directly exposed to the air and externalised.
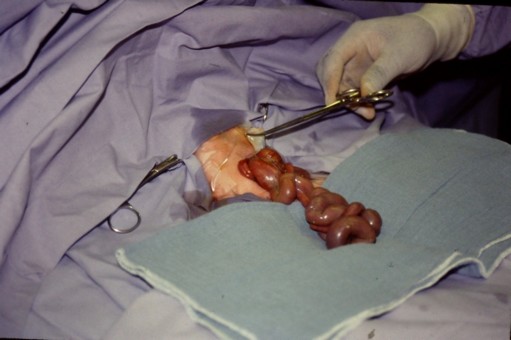
Delivery occurs, on average, in the 35-36th week of gestation often with some intrauterine growth retardation (weight less than the 10th percentile for gestational age in more than 20% of cases). Classically it was deemed that unlike omphalocele, the gastroschisis is rarely associated with other congenital anomalies but recent epidemiological data contradict this point: at least one associated malformation is observed in 32% of cases:
- atresia of the small bowel or colon : the term 'complex gastroschisis' is used in case of associated intestinal atresia .
- biliary tract pathology
- hydrocephalus
- cryptorchidism.
The factors at the origin of the anomaly of parietal closure remain unknown.
Several hypotheses have been raised:
- anomaly of the mesoderm, making it unable to form the abdominal wall;
- amniotic rupture around the umbilical ring allowing the extracorporeal herniation of the abdominal content;
- abnormal involution of the right umbilical vein resulting in parietal weakening and intestinal herniation;
- interruption of the right vitelline artery causing parietal ischemia and then bowel herniation;
- finally, the most recent hypothesis is that abnormal folding of the abdominal wall causes a parietal dehiscence
The diagnosis of gastroschisis is easy to do before birth thanks to echography. However, the specific socioeconomic context of some young mothers leads to not optimal pregnancy monitoring. It results that the diagnosis is made at the time of birth (or just before). When the malformation is recognized during pregnancy, the follow-up must be ensured by a tertiary obstetric center where the mother will be transferred for delivery around the 34-35th week of pregnancy.
The strategy of childbirth varies according to centers:
- elective c-section between the 36th and 38th gestational week,
- induced vaginal delivery at week 36
- ultrasound monitoring and induction of delivery, as soon as a thickening of the externalized small bowel is visible on ultrasound.
As soon as the baby is delivered, care management includes:
- prevention of bacterial contamination, visceral injury and heat loss: the externalized abdominal content should be packed with warm sterile wet gauze sw wrapped in a plastic waterproof bag (or food wrapping film), ensuring that there is no twisting of the vascular vessels nor bleeding at the level of the umbilical cord clamp: this isolates the lower body of the child from the outside environment.
- prevention of hypothermia;
- fluid resuscitation of the child;
- quick transfer to NICU (Neonatal Intensive Care Unit).
Once the child cared in the NICU, the subsequent strategy varies according to the teams and the importance of the lesion.
Several strategies are possible:
- either emergency surgical repair, in the hours following birth, in order to improve the prognosis of the disease
- either gradual reintegration of the loops by the placement, in the ICU, of a silastic ("silo") armed prosthesis with progressive reduction of the externalized abdominal content by placing it, in the ICU, in a silastic ('silo') prosthesis to perform a progressive reduction of the herniation without surgery nor general anesthesia: the results seem to be equivalent to surgical closure, but with a possible benefit of a "trend" to a reduced number of days of ventilation.
A score of prognosis of the gastroschisis has been proposed in Canada: it is based on examination of the intestinal intestinal loops within 6 hours following birth (see table): a score higher than 2 implies an increased morbidity (delay to oral feeding and stop of total parental nutrition) and a score higher than 4 means a high risk of mortality
|
Adhesions |
absent = 0 |
moderate = 1 |
severe = 4 |
|
Atresia |
absent = 0 |
suspected = 1 |
This = 2 |
|
Perforation |
absent = 0 |
|
This = 2 |
|
Necrosis |
absent = 0 |
|
present = 4 |
Score of the gastroschisis
Reintroduction of the intestinal loops into an partially or very hypoplastic abdominal cavity involves three main risks:
- abdominal hypertension impairing ventilation,
- compression of the inferior vena cava, compromising the venous return,
- abdominal compartmental syndrome compromising the vascularization of the abdominal contents (mesenteric but also renal, or hepatic ischemia).
Once the immediate postoperative period is over , the main problems is nutrition due to the fact that the digestive tract is pathological, inflammatory and often of reduced length (short bowel syndrome). A significant number of children who survived the malformation in the neonatal period will need a special food diet lifelong (very constraining), or even parenteral nutrition (with all its possible complications).
Anesthetic implications:
- urgent neonatal surgery
- a central venous catheter allows to evaluate volemia and the impact on the venous return of the reintegration of the intestinal loops, and to ensure postoperative parenteral nutrition.
- an arterial catheter to monitor continuously the hemodynamics, but also to allow blood sampling to monitor blood gases, glucose and electrolytes
- prevention of hypothermia, given the importance of the surface exposed to caloric loss ;
- pre- and postductal monitoring of blood saturation at the level of the upper limbs (right and left side, respectively), given the risk of right-left shunt through a patent foramen ovale and ductus arteriosus; monitoring of the cerebral oxygenation (NIRS)
- rapid sequence induction after emptying gastric contents and assessing the risk of difficult intubation (in the rare case of associated orofacial malformations);
- curarization (ask the surgeon for advice : some fear that muscle paralysis could affect the evaluation of intraabdominal pressure during abdominal closure) and controlled ventilation with a mixture of air and of O2 (maintaining oxygen saturation between 93 and 97 %). Anesthetic maintenance may be based on an association of an opiate (fentanyl, sufentanil, continuous remifentanil) and an halogenated agent.
- infusion of dopamine or noradrenaline is sometimes needed to maintain adequate diuresis and blood pressure.
The most delicate phase is the reintegration of the intestinal loops in the abdominal cavity. Several pressure measurements during a trial of closure of the wall help to evaluate the risk of postoperative abdominal compartment syndrome.
Primary closure is better avoided when:
- intragastric or intravesical pressure is greater than 20 cm H2O when the abdomen is closed; pressure in the stomach can be measured by filling the gastric tube with water and connecting it to a PVC system or to a pressure gauge (same as that used to measure the pressure in the pilot balloon of ETT)
- peek insufflation pressure greater than 30 cm H2O is needed to keep ventilation similar to that before the attempt of closure.
- splanchnic perfusion pressure calculated by subtracting the indirect measured intra-abdominal pressure (i.e., intragastric or intravesical pressure) from the mean systemic blood pressure, is less than 35 mmHg.
When there is a risk of excessive intra-abdominal pressure, the placement of a silastic prosthesis is to be preferred. The silo is kept vertically to avoid any pressure effect of extraabdominal viscera on the abdominal wall. In a 2 to 10 days period of time, the viscera protected by the bag are gradually reintegrated into the abdominal cavity and final skin closure is ultimately performed in the operating room.
In most cases, the neonate is kept intubated and under mechanical ventilation in early postoperative period.
References:
- Vegunta RK, Wallace LJ, Leonardi MR, Gross TL, Renfroe Y, Marshall JS, Cohen HS, Hocker JR, Macwan KS, Clark SE, Ramiro S, Pearl RH.
Perinatal management of gastroschisis: analysis of a newly established clinical pathway.
J Pediatr Surg 2005; 40:528-34.
- Davies MW, Kimble RM, Cartwright DW.
Gastroschisis: ward reduction compared with traditional reduction under general anesthesia.
J Pediatr Surg 2005; 40:523-7.
- Lansdale N, Hill R, Gull-Zamir S, Drewett M, Parkinson E, Davenport M, Sadiq J, Lakhoo K, Marven S.
Staged reduction of gastroschisis using preformed silos: practicalities and problems.
J Pediatr Surg 2009; 44:2126-9.
- Yaster M, Buck JR, Dudgeon DL, Manolio TA, Simmons RS, Zeller P, Haller JA Jr.
Hemodynamic effects of primary closure of omphalocele/gastrochisis in newborns.
Anesthesiology 1988; 69:84-88.
- Rizzo A, Davis PC, Hamm CR, Powell RW.
Intraoperative vesical pressure measurement as a guide in the closure of abdominal wall defects.
Am Surg 1996; 62:192-195.
- Olesevich M, Alexander F, Khan M, Cotman K.
Gastroschisis revisited: role of intraoperative measurement of abdominal pressure.
J Pediatr Surg 2005; 40:789-92.
- McGuigan RM, Mullenix PS, Vegunta R, Pearl RH, Sawin R, Azarow KS.
Splanchnic perfusion pressure: a better predictor of safe primary closure than intraabdominal pressure in neonatal gastroschisis.
J Pediatr Surg 2006; 41:901-4.
- Raghavan M, Montgomerie J.
Anesthetic management of gastroschisis: a review of our practice over the past 5 years.
Pediatr Anesth 2008; 18:1055-9.
- Benjamin B, Wilson GN.
Anomalies associated with gastroschisis and omphalocele: analysis of 2825 cases from the Texas Birth Defects Registry.
J Pediatr Surg 2014; 49: 514-9
- Cowan KN, Puligandla PS, Laberge J-M et al.
The gastroschisis prognostic score : reliable outcome prediction in gastroschisis.
J Pediatr Surg 2012 ; 47 : 1111-7
- Mhamane R, Dave N, Garasia M.
Delayed primary repair of giant omphalocele: anesthesia challenges.
Pediatr Anesth 2012 ; 22 : 935-6
- Burgos CM, Irvine W, Vivanti A, Conner P, Machtejeviene, Peters N et al.
European reference network for rare inherited congenital anomalies (ERNICA) evidence based guideline on the management of gastroschisis.
Orphanet J Rare Diseases 2024 ; 19 : 60. doi.org/10.1186/s13023-024-03062-8
Updated: July 2025