Overall incidence between 2 and 5/10,000 live births. It is isolated in 58 % of cases;
A genetic origin is found in rare cases: DIH1 (acronym for DIaphramatic Hernia) 1 [MIM 142 340], mutation at 15q26, DIH 2 [MIM 222 400], mutation at 8p23, DIH3 [MIM 610 187], mutation of the ZFPM2 gene (8q23. 1), DIH4 [MIM 617,557], ALDH1A2 gene mutation (15q21.3) and an X-linked form [MIM 306,950].
The others are associated with:
- cardiac malformations: ASD, VSD, tetralogy of Fallot, coarctation of the aorta,
- syndromes such as Fryns (10 %), CHARGE, Beckwitt-Wiedemann, Donnai-Barrow, Cornelia De Lange, Denys-Drash, Pallister-Killian, Wolf-Hirshhorn, Simpson-Golabi-Behmel, Kabuki, Mathew-Wood syndrome (see these terms)
- chromosomal abnormalities: trisomy 13, 18 or 21, monosomy X, some deletions or duplications
- a pulmonary malformation: pulmonary sequestration is present in about 3.4 % of cases: the defect of the diaphragm is then often larger (types C or D) and these patients more often need ECMO.
Diaphragmatic hernia results from an abnormality in the closure of the pleuro-pericardo-peritoneal canal, allowing the intrusion and development of abdominal viscera into the chest cavity as early as the 8th week of gestation. In the vast majority of cases, it consists of a hernia through the Bochdalek's foramen: it is
- posterior-lateral on the left side in 75-90 % of cases;
- right-sided in about 10 % of cases;
- bilateral in 1 to 5 % of cases (usually fatal).
In the other situations, a hernia can sometimes occur retrosternally (23 %) or centrally (1-2 %), through the Morgagni's foramen (or the Larrey's cleft).
Diagnosis: most often during pregnancy (ultrasound examination) in developed countries.
Severity depends on several factors:
- the fetal stage at which herniation occurs
- the degree of pulmonary hypoplasia knowing that while lung damage is much more severe on the herniated side, some degree of hypoplasia is also present on the unaffected side;
- the presence of the liver in the chest is an important factor of severity: survival is better if the liver is intraabdominal
- the associated lesions: isolated forms have a better prognosis than the forms associated with other malformations or genetic abnormalities.
The lungs show a decrease in the divisions of the bronchial tree and of the pulmonary artery, especially on the herniated side: this contributes mechanically to an increase in pulmonary resistance and thus blood pressure. The herniation of abdominal viscera into the chest also affects the anatomical and physiological development of the heart, in isolated forms as well as in non-isolated forms. There is often a relative hypoplasia of the left ventricle, the filling of which is impaired as a result of alterations of the flow in the ductus venosus by the herniated organs. However, these abnormalities normalize quickly after the surgical repair of the hernia, allowing normal biventricular circulation. Moreover, many children have adrenal deficiency that significantly worsens respiratory morbidity and could explain their frequent resistance to treatment with catecholamines.
Even in the absence of a cardiac defect, the arterial canal and the foramen ovale are not anatomically closed at birth and the hemodynamic and biological consequences (hypoxemia, hypercarbia, acidosis) of respiratory failure promote their (re)opening: a right-left shunt may occur, aggravating the hypoxemia due to pulmonary hypoplasia. However, a left-right shunt is not uncommon either at the atrial level (due to decreased compliance of the LV), either in case of a right-left shunt at the ductal level (pulmonary arterial hypertension).
The best prognosis indicators currently selected are:
- the weight of the fetus at birth (above or below 2750 g),
- the presence of an intrathoracic hernia of the liver,
- the presence of associated malformations
- one echographic criteria: the relationship between the surface of the lung and the circumference of the head; this O/E LHC ratio (Observed/Expected Lung area to Head Circumference ratio) is expressed as a percentage of the expected normal ratio for the fetal age. According to this ratio, the case is defined as extreme (< 15 %), severe (16-25 %), moderate (26-35 %) or mild (36-45 %) . In case of isolated left diaphragmatic hernia, the survival rate is null in case of extreme form, around 20 % in case of severe form, 55 % in case of mild form and 85 % in case of minor form.
When prognostic indicators are poor, which means,
- in case of left hernia: O/ELHC ratio < 25 % or between 25 and 35 %, whatever the position of the liver, or 35 to 45 % with intrathoracic herniation of the liver
- in case of right hernia: O/ELHC < 45
- in case of bilateral herniation: O/ELHC < 25 %.
some teams try to promote lung growth by occluding the fetal trachea using percutaneous intrauterine endoscopy ("FETO" for Fetal Endoscopic Tracheal Occlusion). This technique consists of introducing percutaneously in the sedated future mother a vascular occlusion cuff into the foetus’ trachea via the translaryngeal route between the 26th and 28th week of gestation.
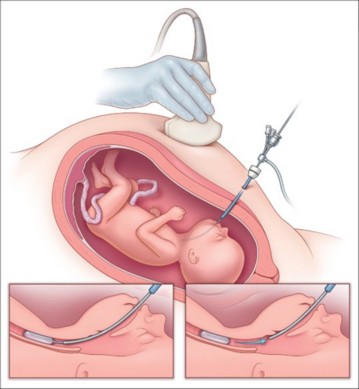
This balloon (7-8 mm in diameter x 20-22 mm long) is kept inflated (0.6 ml of 0.9 % NaCl) until around the 34th week: intratracheal pressure reduces the number of type II pneumocytes, thus exposing the patient to the risk of surfactant deficiency. Removal of the balloon is performed by percutaneous endoscopy or puncture under ultrasound guidance. This technique gives a survival rate of around 50 % in severe cases. Survival is not improved in moderate cases. When the balloon is removed, the risk of premature birth is multiplied by 2.6 in severe cases and by 2.9 in moderate cases.
The diameter of the trachea is increased by an average of 31 % in newborns (tracheomegaly) who have benefited from a FETO, and they present an additional risk (around x5) of tracheomalacia (hyperlaxity of the pars membranacea).
Diagnosis at birth:
When the diagnosis is not made before birth, the clinical symptomatology presented by the newborn depends on the degree of pulmonary hypoplasia, the mass of herniated viscera, the degree of mediastinal deviation, the presence of an ileus and intestinal dilation, and ultimately the importance of pulmonary arterial hypertension. In the usual form, the newborn develops rapidly tachypnea, dyspnea and cyanosis after birth.
In addition to the signs of respiratory distress, physical examination shows:
- an unilateral decrease or absence of breath sounds
- tympanism of the hemithorax with the hernia;
- and a depressed hollow, 'scaphoid' abdomen
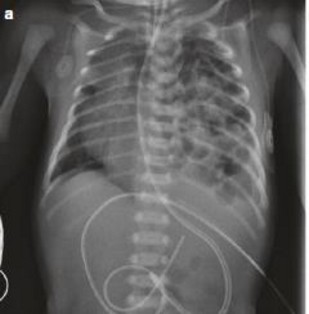
Heart sounds are muffled and moved toward the side opposite to the hernia. Peristalsis can sometimes be heard in the chest. A chest X-ray show intestinal loops in the hemithorax, a controlateral mediastinal deviation, poor visibility of lung tissue and, sometimes, an intrathoracic loop of the gastric tube (wrapped in a stomach partially or entirely located in the chest).
To treat the respiratory distress, the initial step is ventilatory assistance with immediate intubation (if possible without positive pressure bag-mask ventilation to avoid insufflating the intrathoracic stomach), oxygenation and gastric decompression. Thereafter, the management is the same as in the forms diagnosed before birth (see below).
Some forms, associated with minimal pulmonary hypoplasia, are not symptomatic at birth but diagnosed later. They are discovered during an episode of dyspnea (e.g. during a respiratory infection) or by chance during a chest X-ray. These are most often anterior hernias through the Morgagni's foramen but some hernias through the Bochdalek foramen may also present much later in life.
Programmed management: in a tertiary centre.
Prognostic criteria are regularly reassessed and prenatal interventions may be performed (see above). Birth is usually planned around the 36-38th week in collaboration with the team of neonatologists who will take care of the child. The newborn is intubated immediately after birth (often before clamping of the umbilical cord), given analgesics, paralyzed and transferred to the NICU where it will be placed under non-conventional ventilation using small volumes and rapid ventilation rate (PIP < 25 cm H2O, 40-60/min) (to limit pulmonary barotrauma and risk of pneumothorax), and tolerating permissive hypercapnia (paCO2 50-70 mmHg). The goal is to improve oxygenation (Fi02 titration to reach a preductal Sp02 between 80 and 95 %), to correct acid-base imbalances, to reduce right-left shunt and to improve the pulmonary perfusion by controlling lung arterial hypertension. In case of failure of conventional ventilation or from the outset in some units, high-frequency or oscillation ventilation is used (mean pressure 14-16 cm H20, ΔP 30-40 cm H20), introducing NO if preductal Sp02 is low as in case of atrial right-to-left shunt (but to be avoided in case of left-right shunt at the atrial level !), with the aim of increasing pulmonary perfusion as well as systemic oxygenation. In case of dysfunction of the LV, milrinone can be useful. There is often a period of relative improvement and stabilization of the child ("honeymoon") during the first 24 hours before a possible secondary, sometimes lethal, deterioration.
According to the preference of the team, early surgery is either carried out by deliberately leaving the ductus arteriosus open (prostacycline infusion) to serve as a discharge valve in case of pulmonary hypertension crisis, or surgery is delayed for a few days until the child is stabilized (hemodynamic and respiratory stability), to allow the development of some pulmonary maturation and a decrease in pulmonary arterial pressure.
The operability criteria recommended in Europe are:
- a normal MAP for age (45 mmHg)
- a preductal SpO2 between 85 to 95 % with a FiO2 < 50 %
- a blood lactates level < 3 mmol/L
- an urinary flow > 1 ml/kg/h
If the child's condition does not stabilize or, a fortiori, if it deteriorates, many teams then consider, after a variable period, the use of extracorporeal membrane oxygenation (ECMO).
The criteria for setting up an ECMO are usually based on:
- a PaCO2 > 67 mmHg despite optimal ventilation (PIP > 28 cm H2O on conventional ventilation)
- a preductal SpO2 < 80% or a postductal SpO2 < 70%
- a PH < 7.15 and blood lactate levels > 5 mmol/l
- a refractory systemic hypotension despite volume loading and hemodynamic support with inotropes, and an urinary flow < 0.5 ml/kg/h
- inability to lower the oxygenation index to less than 30 (oxygenation index = mean ventilation pressure x FiO2/PaO2 x 100),
Classic open surgery
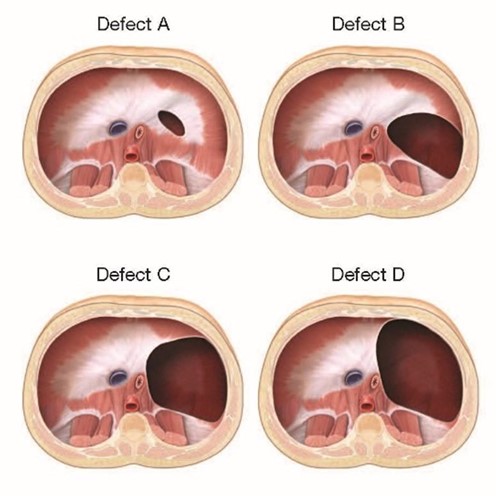
Sub-costal abdominal incision on the involved side (left most often) and reintegration of the herniated viscera into the abdominal cavity followed by diaphragmatic repair: according to the size of the defect, the diaphragm is repaired either directly (type A or B defects) or with a patch (Goretex) (type C or D defects). The recurrence rate is high (46 %) in case of patch repair (regardless of the material used).
|
International classification of diaphragmatic hernias A: small, muscle-only defect B: 50-75 % of the diaphragm is present, the defect includes < 50 % of the chest wall C: 25 % of the diaphragm is present, the defect includes > 50 % of the chest wall D: little or no diaphragm (agenesis)
|
Sometimes the importance of intrathoracic abdominal content requires a combined approach, thoracic first followed by an abdominal one. The abdominal cavity is hypoplastic and can make closure difficult: it is not uncommon to have to place transiently a silastic parietal prosthesis to avoid:
- intra-abdominal hyperpressure (which can be estimated by measuring intragastric or intra-bladder pressure, which should both normally be less than 20 cm H2O) with all the risks of abdominal compartment syndrome (intestinal ischemia, renal failure),
- decrease in chest compliance.
A non-aspirative chest drain is often placed on the operated side to allow air and secretions drainage without causing any mediatinal displacement.
The complexity of the anesthetic management depends on the state of hemodynamic stabilization (pulmonary blood pressure above or below systemic pressure) and the modalities of ventilation of the child in the immediate preoperative period. Transferring the child to the operating room is a delicate step. In principle, the child arrives with a nasotracheal tube in place and is ventilated, either mechanically or, more often, manually throughout the transfer phase.
If the child is under conventional ventilation, it is best pursued intraoperatively with the aim of maintaining the preoperative PaO2 and PaCO2 values with, ideally, an arterial saturation greater than 90 % (or a PaO2 > 60 mmHg), trying to avoid any episode of major hypercapnia with a consequent increased pulmonary resistance and right-left shunt.
Anesthesia can be maintained with:
- low concentration of sevoflurane
- titrated morphinic (sufentanil)
- deep curarization by a non-depolarizing agent
If the child receives NO, it should be continued intraoperatively. Some teams use a specific injector (INOvent®). If it is used with a circle circuit, this injector must be placed on the inspiratory side of the circuit, downstream of the inspiratory valve and CO2 absorber. Most teams, however, use the ventilator on which the child was connected in the NICU: this requires using a total intravenous anesthesia technique, as halogenated anesthetics evaporators cannot be connected to this type of ventilator. PaCO2 should be controlled very closely due to the large variations in chest compliance during the hernia reduction phase and the risk of cerebral hypoperfusion in case of severe hypocapnia.
If the child is on ECMO, the difficulties are mainly related to anticoagulation.
Surgical correction forms with a late diagnosis is the same, but anesthetic management is simpler because there is no problem with ventilation or oxygenation. The use of N2O is contraindicated and, in addition to the usual monitoring, it is useful to put an arterial line for invasive blood pressure monitoring and regular arterial gases, specifically PaO2 and PaCO2.
In case of gastric distension, it is imperative to decompress the stomach before anesthesia induction or immediately after intubation (if necessary through emergency transparietal needle drainage) to avoid compression of the heart and large vessels that can cause shock or even cardiac arrest. In these forms, it is interesting to provide epidural analgesia as this technique ensures excellent per and postoperative analgesia.
Laparoscopic surgery
The indications of so-called "minimally invasive" surgery, mainly by laparoscopy, thoracoscopy or the combination of both (often by converting a laparoscopy into thoracoscopy) get more and more frequent. Most teams wait 1 to 3 days before operating ie until the pulmonary systolic blood pressure (estimated by ultrasound) becomes less than 2/3 of the systemic systolic blood pressure.
In practice, laparoscopic surgery works well in children whose respiratory condition is not too severe and the morbidity is lower than that of open surgery. However, the rate of early recurrence is higher than in open surgery, especially if a patch had to be placed to close the diaphragmatic defect. Anesthetic management of laparoscopy is not significantly different from that of open surgery, except for severe hypercapnia (with respiratory acidosis), more frequent episodes of cerebral hypoxia, and often longer duration of surgery (risk of hypothermia).
Surgical and later outcome
In the postoperative period, there is often a transient deterioration in ventilation due to postoperative decrease in thoracic compliance. Despite improved management, mortality among children with diaphragmatic hernia remains high, at around 20 % for favorable cases in the best centres, but reaching or exceeding 50 % in the most severe forms, notably those associated with malformations and/or requiring ECMO treatment. The recurrence rate requiring re-intervention is in the order of 10 % in case of primary repair of the diaphragm but 50 % if a patch (especially if it is an absorbable patch) was used. Other most common later complications include:
- chest deformities (46 %), more frequent after high prematurity, patch use, intrathoracic gastric hernia, prolonged post-surgical ventilation, long-term O2 requirement;
- intestinal obstruction (13 %), even more common when a patch is needed;
- scoliosis (13 %);
- chronic respiratory problems: bronchodysplasia
- regarding the pulmonary hypertension
1. some children do not present any kind of PH and have a excellent prognosis
2. some others present with transient PH that decreases in 4 to 6 weeks (treatment by sildenafil);
3. some others keep a nearly systemic PH; a cardiac catheterism is necessay to search for an anomaly of the pulmonary arteries, of the pulmonary veins or of the LV: in case of anomaly of the left heart, those patients can benefit from the addition of milrinone or an ACE inhibitor; otherwise, the pulmonary vasodilator treatment should be strengthened; their prognosis is very poor.
A significant number of children with diaphragmatic hernia require gastric fundoplication. Moreover, there is a progressive diminution of the ventilation/perfusion ratio with growth (1.58 at 1 year of age → 1.82 at 15 years of age). It is due to a decrease of the perfusion on the hernia side. The larger the repair, the more important is the diminution of perfusion (type C or D defects).
Anesthetic implications:
neonatal surgery, pre- and post-ductal SpO2, arterial line, monitoring of brain oxygenation; difficult ventilation; hemodynamic support for hypotension; dopamine or norepinephrine, sometimes dobutamine. Preoperative echocardiography: pulmonary blood pressure (shift of the interventricular septum, right-left shunt at the level of the ductus arteriosus), change ofdirection of flow in the foramen ovale, associated cardiac disease, LV function ? Pulmonary vascular hyperreactivity is unpredictable and can result in sudden pulmonary hypertension. Measuring intragastric or intravesical pressure if the abdomen is difficult to close.
References :
- Lally KP, Lasky RE, Lally P, Bagolan P et al.
Standardized reporting for congenital diaphragmatic hernia - an international consensus.
J Pediatr Surg 2013; 48:2408-15.
- Doné E, Gucciardo L, Van Mieghem T, Jani J, Cannie M, Van Schoubroeck D, Devlieger R, Catte LD, Klaritsch P, Mayer S, Beck V, Debeer A, Gratacos E, Nicolaides K, Deprest J.
Prenatal diagnosis, prediction of outcome and in utero therapy of isolated congenital diaphragmatic hernia.
Prenat Diagn 2008; 28:581-91.
- Vogel M, McElhinney DB, Marcus E, Morash D, Jennings RW, Tworetzky W.
Significance and outcome of left heart hypoplasia in fetal congenital diaphragmatic hernia.
Ultrasound Obstet Gynecol 2010; 35:310-7.
- Kamath BD, Fashaw L, Kinsella JP.
Adrenal insufficiency in newborns with congenital diaphragmatic hernia.
J Pediatr 2010; 156:495-7.
- Hirose S, Farmer DL, Lee H, Nobuhara KK, Harrison MR.
The ex utero intrapartum treatment procedure: Looking back at the EXIT.
J Pediatr Surg 2004; 39:375-80.
- Keller RL, Hawgood S, Neuhaus JM, Farmer DL, Lee H, Albanese CT, Harrison MR, Kitterman JA.
Infant pulmonary function in a randomized trial of fetal tracheal occlusion for severe congenital diaphragmatic hernia.
Pediatr Res 2004; 56:818-25.
- Harrison MR, Keller RL, Hawgood SB, Kitterman JA, Sandberg PL, Farmer DL, Lee H, Filly RA, Farrell JA, Albanese CT.
A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia.
N Engl J Med 2003; 349:1916-24.
- Bösenberg AT, Brown RA.
Management of congenital diaphragmatic hernia.
Curr Opin Anaesthesiol 2008; 21:323-31.
- Congenital Diaphragmatic Hernia Study Group, Bryner BS, West BT, Hirschl RB, Drongowski RA, Lally KP, Lally P, Mychaliska GB.
Congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation: does timing of repair matter?
J Pediatr Surg 2009; 44:1165-71.
- Harting MT, Lally KP.
Surgical management of neonates with congenital diaphragmatic hernia.
Semin Pediatr Surg 2007; 16:109-14.
- Migliazza L, Bellan C, Alberti D, Auriemma A, Burgio G, Locatelli G, Colombo A.
Retrospective study of 111 cases of congenital diaphragmatic hernia treated with early high-frequency oscillatory ventilation and presurgical stabilization.
J Pediatr Surg 2007; 42:1526-32.
- Kutzsche S, Sangolt GK, Schistad O, Sunde S.
Severe complications during the management of a child with late presentation of a diaphragmatic hernia.
Acta Anaesthesiol Scand 2003; 47:1302-4.
- Gourlay DM, Cassidy LD, Sato TT, Lal DR, Arca MJ.
Beyond feasibility: a comparison of newborns undergoing thoracoscopic and open repair of congenital diaphragmatic hernias.
J Pediatr Surg 2009; 44:1702-7.
- McHoney M, Giacomello L, Nah SA, De Coppi P, Kiely EM, Curry JI, Drake DP, Eaton S, Pierro A.
Thoracoscopic repair of congenital diaphragmatic hernia: intraoperative ventilation and recurrence.
J Pediatr Surg 2010; 45:355-9.
- Deprest J, Breysem L, Gratacos E, Nicolaides K, Claus F t al.
Tracheal side effects following fetal endoscopic tracheal occlusion for severe congenital diaphragmaic hernia.
Pediatr Radiol 2010; 40: 670-3
- Snoek KG, Reiss IKM, Greenough A, Capolupo I, Urlesberer B et al.
Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium Consensus -2015 Update.
Neonatology 2016; 110: 66-74.
- Quinney M, Wellespey H.
Anaesthetic management of patients with a congenital diaphragmatic hernia.
BJA Education 2018; 18: 95-101.
- Wehrmann M, Patel SS, Haxel C, Cassidy C, Howley L, Cunoe B, Gien J, Kinsella JP.
Implications of atrial-level shunting by echocardiography in newborns with congenital diaphragmatic hernia.
J Pediatr 2020; 219: 43-7
- Dao DT, Kamran A, Wilson JM, Sheils CA et al.
Longitudinal analysis of ventilation perfusion mismatch in congenital diaphragmatic hernia survivors.
J Pediatr 2020; 219:160-6
- Chatterjee D, Ing RJ, Gien J.
Update on congenital diaphragmatic hernia.
Anesth Analg 2020; 131: 808-21.
- Coughlin MA, Gupta VS, Ebanks AH, Harting MT et al.
Incidence and outcomes of patients with congenital diaphragmatic hernia and pulmonary sequestration.
J Pediatr Surg 2021; 56:1126-9.
- Deprest JA, Nicolaides KH, Benachi A, Gratacos E, Ryan G et al.
Randomized trial of fetal surgery for severe left diaphragmatic hernia.
N Engl J Med 2021; 385:107-18
- Deprest JA, Benachi A, Gratacos E, Nicolaides KH, Berg C et al.
Randomized trial of fetal surgery for moderate left diaphragmatic hernia.
N Engl J Med 2021; 385:119-29
Updated: September 2024