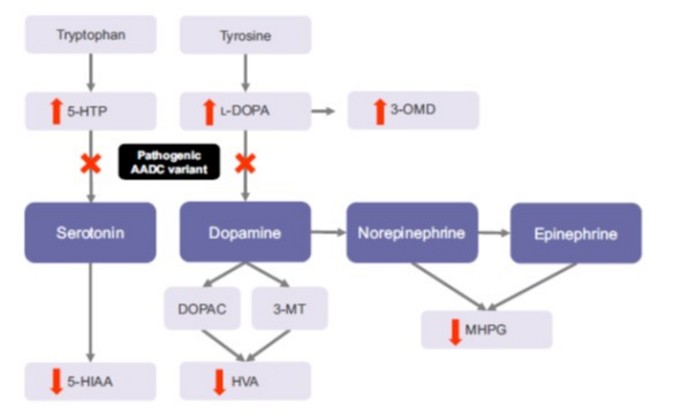
Very rare: about 100 cases have been described, mostly in patients from South China (Taiwan) and Japan. Autosomal recessive transmission of a mutation of the SDC gene (17p12.2-p12.1) coding for the aromatic L-amino acids decarboxylase: this pyridoxine-dependent enzyme is necessary for the decarboxylation of levodopa into dopamine (stage of the synthesis of adrenaline and norepinephrine) and of 5-hydroxytryptophan into serotonin. The dysfunction of this enzyme leads to a deficiency in dopamine, epinephrine and norepinephrine, and serotonin.
The clinical picture varies widely:
- onset of the first signs during the first months of life: hypotonia, oculogyric crises, dystonia, hypokinesia, vegetative system disorders;
- developmental retardation
- predominance of the vagal tone: bradycardia, sweating, ptosis, myosis, nasal congestion, low blood pressure, gastroesophageal reflux
- body temperature control disorder: hypo- or hyperthermia
- irritability, excessive crying, autistic behavior
- stridor secondary to laryngo- or tracheomalacia
- dystonic seizures consist of intense muscle contractions that can lead to respiratory or metabolic disorders (rhabdomyolysis)
Depending on the clinical presentation, several forms can be identified:
- a severe form: severe developmental retardation, totally dependent child
- a moderate form: mild developmental retardation, walking without assistance
- an intermediate form
Diagnosis: very low levels of catecholaminergic neurotransmitters and their metabolites in CSF.
Treatment is symptomatic and needs to be individualized.
It generally associates:
- a dopamine receptor agonist: for example, pramipexole (dopamine receptor agonist): 6 to 35 µg/kg/d in 2 intakes.
- a MAO B inhibitor: selegiline from 0.1 to 0.25 mg/kg/d in 3-4 intakes
- pyridoxine or pyridoxal phosphate, a cofactor of the aromatic L-amino acids decarboxylase
And sometimes:
- a central anticholinergic: benztropin, trihexyphenidal in 2 to 4 doses per day, in case of dystonia or significant Parkinson's signs
- melatonin in case of sleep disturbances, 3 mg at bedtime
- folinic acid: 1 to 2 mg/kg/d (maximum 20 mg)
- and, only in certain mutations: L-Dopa not associated with a dopa-decarboxylase inhibitor: 0.5 to 5 mg/kg/d in 3 intakes
5-hydroxytryptophan and serotonin reuptake inhibitors, as well as central dopamine antagonists such as haloperidol and metoclopramide, should be avoided.
Caution with the use of the 5HT3 serotonin antagonists (ondansetron etc).
Experimental gene therapy: stereotactic (unique and bilateral) injection (MRI) of an adenovirus-associated vector (eladocargene, exuparvovec, AAV2- Upstaza®) containing the human PTC-AADC gene into the dopaminergic neurons of the putamen in children aged > 18 months.
Anesthetic implications:
Significant drooling. Difficult peripheral venous access. Continue medical treatment until the day of the procedure; recent ECG and echocardiography; risk of hypoglycemia; decreased response to hypovolemia: in case of hypotension, there is no response to ephedrine and the response to direct amines is often excessive;it seems preferable to use titrated doses of dopamine or noradrenaline; avoid central dopamine antagonists such as metoclopramide and butyrophenones. Atropine to counteract the predominant vagal tone and avoid bradycardia ?
Risk of hypo- or hyperthermia with profuse sweating.
Drug interactions: selegiline (MAO B inhibitor) does not in principle interact with the anesthetic agents if the total dose is less than 10 mg/d but would behave like an MAOI at higher doses.
It is not known whether setrons (5HT3 inhibitors) can be used as antiemetics. It is best to avoid tramadol because of its monoaminergic mode of action.
References :
- Vutskits L, Menache C, Manzano S, Haenggeli CA, Habre W.
Anesthesia management in a young child with aromatic l-amino acid decarboxylase deficiency.
Pediatr Anesth 2006; 16: 82-4.
- Berkowitz DH, Ganesh A.
Combined general and regional anesthetic in a child with aromatic L-amino acid decarboxylase deficiency.
Anesth Analg 2006;103:1630-1.
- Anselm IA, Darras BT.
Catecholamine toxicity in aromatic L-amino acid decarboxylase deficiency.
Pediatr Neurol 2006; 35: 142-4.
- Wassenberg T, Molero-Luis M, Jeltsch K, et al.
Consensus guideline for the diagnosis and treatment of aromatic l-amino acid decarboxylase (AADC) deficiency.
Orphanet J Rare Dis 2017;12:12.
- Viviani C, Buelli E, Fierro G, Stiffan S, Lorini FL.
Anesthesia management for cesarean delivery in a woman with aromatic l-amino acid decarboxylase deficiency : a case report.
A&A Practice ; 2020 ; 14 : e01275
- McCarthy A, Black C.
Anaesthesia management of a child with aromatic L-amino acid decarboxylase deficiency.
Anaesthesia Reports 2022 ; 10 : e12152
- Roubertie A, Opladen T, Brennenstuhl H, et al.
Gene therapy for aromaticL-amino acid decarboxylase deficiency: requirements for safe application and knowledge-generating follow-up.
J Inherit Metab Dis 2023;1‐13. doi.org/10.1002/jimd.12649
- Kanjia MK, Jooste EH, Illig M, et al.
Optimizing the anesthetic care of patients with aromatic l-amino acid decarboxylase deficiency.
Pediatr Anesth 2025; 35:99-106 doi:10.1111/pan.15025
- Clariot S, Gras S, Goetz L, Boulloud C, Bonheur J, Vayssiere P, D'Hardemare V, Ravelli C, Dorison N, Devys J-M.
Case series of anesthetic management of gene therapy in children with aromatic L-Amino Acid Decarboxylase deficiency.
Pediatr Anesth 2025; 35:316-20
Updated: March 2025